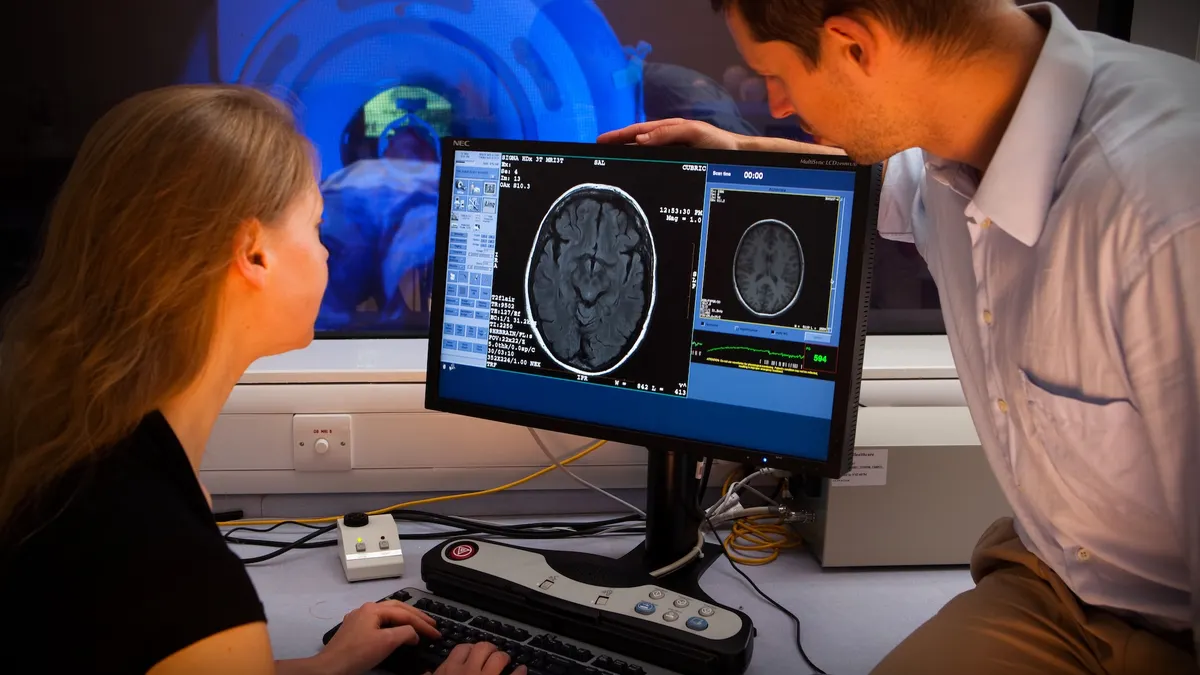
Biotechnology companies specializing in psychedelics research saw their share prices rise after rumors of a billion-dollar acquisition hinted that big pharma is now more open to betting on this area of drug development.
Bloomberg News reported early Thursday that AbbVie is in talks to buy privately held Gilgamesh Pharmaceuticals. If agreed to, the deal would hand AbbVie a small slate of experimental therapies for depression, anxiety and mental health conditions. Gilgamesh’s most advanced drug, code-named GM-2505, works by latching onto a brain protein known to interact with psychedelics like LSD and psilocybin.
Gilgamesh’s possible takeover appears to have buoyed investor confidence in psychedelics companies. Shares of Cybin, Mind Medicine and GH Research — which are respectively advancing their own versions of psilocybin, LSD, and a psychoactive compound found in certain plants and toads — were up about 3% to 5% by mid-morning Thursday.
Two other biotechs, Atai Life Sciences and Compass Pathways, had gains of more than 10%, though Compass’ later dwindled.
Joshua Schimmer, an analyst at the investment bank Cantor Fitzgerald, said he’s not surprised to see big pharma taking more interest in psychedelics, since these therapies have shown “transformational” potential across a range of mental health disorders. Compass, for one, in June announced results from a late-stage clinical trial wherein a single dose of its psilocybin therapy significantly reduced symptoms of treatment-resistant depression.
Gilgamesh also recently disclosed positive results from a study of GM-2505, which, according to the company, was able to quickly, effectively and durably treat the most common form of depression.
“This is one of the most important waves of innovation we're seeing today in biotech,” Schimmer said. “We need more clinical data readouts in proper, large, randomized, controlled trials. But so far, we're seeing things we've really not seen before in psychiatric care, and it doesn't end with depression.”
Analysts note, too, the inroads psychedelics are making with drug regulators. Martin Makary, commissioner of the Food and Drug Administration, and Robert F. Kennedy Jr., head of the Department of Health and Human Services, both support speeding up the testing — and possible approval — of psychedelics. The FDA, under former president Joe Biden, also issued guidance in 2023 for psychedelic drug developers.
“The macro backdrop continues to improve,” wrote Jefferies analyst Andrew Tsai in a note to clients. Tsai added that a Gilgamesh purchase, should it happen under the alleged terms, would be larger than previous deals for these kinds of assets. That could help assure Wall Street that psychedelics are “an investable space.”
Additionally, concerns raised by last year's flame-out of one of the field's leading players, Lykos Therapeutics, have since eased. Analysts now see the problems that company encountered in its attempt to win approval of MDMA-assisted psychotherapy as unique.
While those tailwinds can help catalyze bigger-ticket acquisitions, there are reasons to believe such deals won’t come easily. For instance, many of the deepest-pocketed drug firms have found neuroscience too risky and backed away from it over the years. That aversion, if it persists, could keep the pool of potential buyers narrow.
Psychedelic developers may also not be so keen to come to the negotiating table, especially since medium-sized companies like Intra-Cellular Therapies, Axsome Therapeutics and Acadia Pharmaceuticals have demonstrated that brain drugs can be successfully commercialized without big pharma resources.
Many of the current crop of psychedelics biotechs have “very strong management teams” with considerable experience selling medicines, Schimmer said. “I don't see why you need a large pharma company to get this done.”
Kabir Nath, CEO of Compass, told BioPharma Dive this week that his team isn’t designing the company to be sold. The focus, rather, is getting Compass’ psilocybin therapy through the final stages of testing and onto the market.
“It's very exciting to see the resurgence of interest in psychiatry in general, from big pharma and so on,” he said, but “we can't expect to be bought or partnered.”
“Like any board, if somebody comes knocking at the right point, we'll have the discussion,” Nath added. “The door is not closed.”
AbbVie has been more active than many of its peers inking deals that revolve around the brain. In late 2023, the company agreed to spend almost $9 billion to take control of Cerevel Therapeutics and a once-promising schizophrenia therapy.
Since then it dropped $1.4 billion on a Johnson & Johnson-backed startup built around a type of brain drug technology. Then, a couple months ago, it took a $355 million stake in a biotech developing genetic medicines for neurological disorders, among other diseases.
A Gilgamesh acquisition would further diversify the company’s neuroscience research while thrusting it into a field few other large pharmaceutical firms have felt comfortable dedicating significant resources to. J&J is the main exception there. It already sells Spravato, a form of ketamine that’s used to treat depression and last year generated more than $1 billion.
J&J also this spring closed its $15 billion purchase of Intra-Cellular, providing access to a lucrative, mind-stabilizing drug called Caplyta as well as a series of experimental, non-hallucinogenic psychedelics.
Analysts at RBC Capital Markets, in a report published last week, argued the psychedelic space is “approaching a tipping point” as late-stage readouts near and Spravato gets on track to deliver $2 billion in yearly sales. Spravato has “increasingly validated the market,” according to the report.
Gavin Clark-Gartner, an analyst at Evercore ISI, agrees the Spravato story has been a major force toward de-risking psychedelic drug research. However, he cautions that the biotechs coming up in the space will likely have to build upon the commercial infrastructure J&J laid.
“It is not necessarily flipping a switch and a one-for-one translation from Spravato to a lot of these other therapies,” he said.