Pittsburgh-based pharmaceutical firm Viatris on Thursday said a pain drug it’s been developing succeeded in two large clinical trials, setting the stage for an approval filing later this year.
The drug is a reformulated version of an old medication, meloxicam, designed to more quickly treat the sharp, “acute” pain felt after an injury or operation. Researchers evaluated the drug in a dental pain study in 2022. Not long after, a pair of late-stage clinical trials began assessing it in nearly 1,000 people who had just undergone either a bunion removal or a hernia repair surgery.
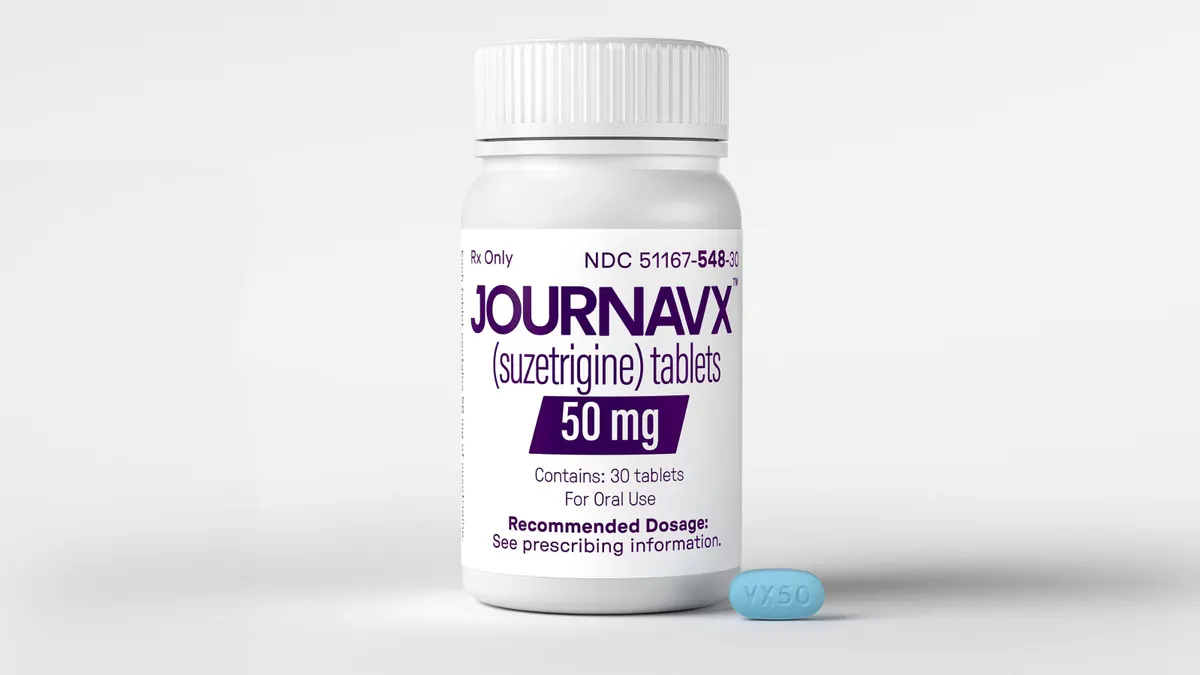
Results from those trials, according to Viatris, now show that pain scores improved substantially more for participants given the drug rather than a placebo. The main goal of both experiments revolved around a scoring system that measures pain intensity over a two-day period. It’s the same system Vertex Pharmaceuticals used in the studies that ultimately led to the approval of Journavx, a closely watched, non-opioid pain reliever.
Viatris, like Vertex, also set up arms in its trials to compare the relief provided by its drug to that of an opioid. The company said after-the-fact analyses found the drug offered “significantly superior pain control” to tramadol. The amount of time it took for participants to sense some pain reduction and to classify that reduction as meaningful was also “comparable” between the two groups — and, in some cases, shorter for those on Viatris’ therapy.
Journavx did not significantly outperform the opioid arms in Vertex’s studies, though those used a combination of Tylenol and a more potent opioid, hydrocodone.
Additionally, Viatris said that during the main treatment phase of its studies, there was a “notable reduction in opioid usage” as well as a higher number of opioid-free patients in the drug group as opposed to the placebo group. Patients also generally tolerated the drug well. There were few so-called severe treatment emergent adverse events, and the incidence of them was comparable across the two groups.
All this evidence “optimally positions” Viatris’ drug to become a first-line treatment for moderate-to-severe acute pain, according to Philippe Martin, the company’s research and development head. "The data observed ... is a critical step in the development of a safe and effective non-opioid option to address an important public health need,” he said in a statement.
The trial results were released alongside Viatris’ first quarter earnings report. The company, which officially formed in 2020, when Mylan merged with Pfizer’s Upjohn unit, recorded $3.3 billion between January and March, an 11% decrease from the same period a year prior. Viatris shares were up over 10% late Thursday morning, to trade around $9.48 apiece.
Shares of Vertex, meanwhile, were down more than 2%, capping off a week in which the company lost tens of billions of dollars in value thanks, in part, to an earnings report that disappointed investors.
Michael Yee, a Jefferies analyst who covers Vertex, wrote in a note to clients that first quarter revenue and earnings per share were “light.” But what stood out was the company saying Journavx revenue was "insignificant” during the period, even though cumulative prescriptions had grown to around 25,000 by late April.
Brian Skorney, a Baird analyst who also follows Vertex, wrote in his own note that the new Viatris data “highlight a number of concerns we have with Journavx’s profile, in particular, onset of action, a critical factor for post-operative pain.”
Skorney went on to suggest the respective bunionectomy studies make Journavx “look like an inferior option” compared to a “pretty old” — albeit new and fast-acting — non-steroidal anti-inflammatory drug, or NSAID.
“If NSAID's don't have the highest efficacy, as Vertex is fond of saying, what does that say about Journavx?” he wrote.