Introduction
Tissue-based comprehensive genomic profiling (CGP) is a cornerstone of precision oncology, enabling the identification of genomic alterations that guide personalized treatment. While it has advanced personalized medicine and precision therapeutic development, its use can be limited by sample availability and procedural constraints. Liquid biopsy offers a complementary, non-invasive alternative that expands access to testing and supports monitoring applications. When used together, a paired tissue-plasma approach enhances flexibility in clinical trial design, improves patient stratification and therapy selection, and strengthens companion diagnostic (CDx) development strategies. This dual-modality framework reflects real-world patient diversity and drives greater efficiency in oncology drug development.
Why tissue-based comprehensive genomic profiling (CGP) matters

Expanding access to CGP with liquid biopsy
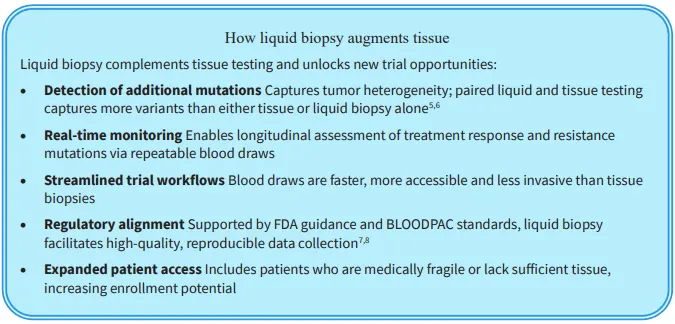
Despite its strengths, tissue-based testing is invasive and often limited by sample availability. Liquid biopsy is a promising, non-invasive option that is increasingly supported for CGP.3, 4 It complements tissue testing in cases of sample insufficiency and broadens access to testing. In addition, it expands the applications of CGP beyond diagnosis and subtyping to longitudinal monitoring of treatment response, tumor resistance and disease recurrence.
A strategic advantage for sponsors—companion diagnostics
When developed as a companion diagnostic (CDx) for your therapy, Labcorp’s paired tissue and liquid biopsy assays can accelerate clinical trials by expanding the eligible patient population and enabling more flexible trial designs—not limited to tissue-only strategies. A paired approach enhances geographic reach to support faster enrollment and provides deeper insights into trial participants through diagnostic subtyping, longitudinal monitoring and detection of tumor resistance and disease recurrence. Moreover, this dual-modality approach helps build a differentiated CDx strategy that better reflects real-world patient diversity and supports faster regulatory approvals.
Labcorp solutions for precision oncology development
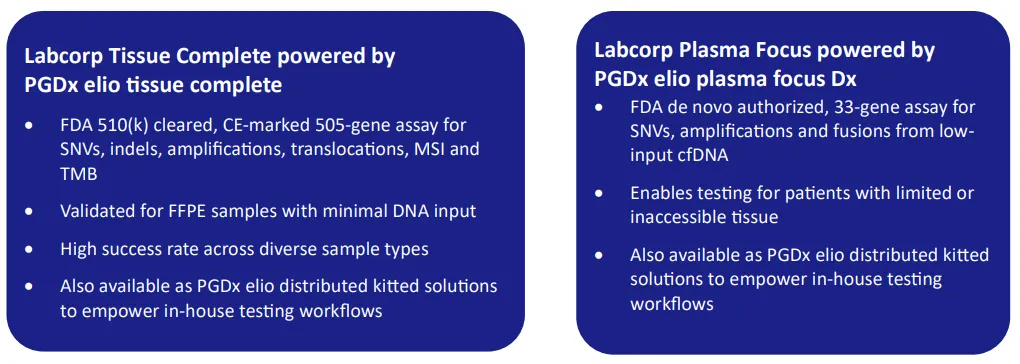
Labcorp has built the validated, high-sensitivity Tissue Complete and Plasma Focus platforms to support this dual-modality strategy. Offering broad genomic coverage and robust, regulatory-grade performance, these solutions are available in our Baltimore, Maryland, U.S., Geneva, Switzerland, and Shanghai, China labs to enable integrated biomarker testing for global clinical trials.
The integration of tissue and plasma-based CGP represents a forward-thinking approach to oncology clinical trials. By leveraging both modalities, sponsors can overcome logistical and biological barriers, improve patient enrollment and streamline regulatory pathways. This approach enhances trial design and better reflects the diversity and complexity of real-world patient populations. As oncology trials evolve, a paired tissue-plasma strategy will be key to driving innovation, expanding access and delivering precision medicine to more patients.
Learn more about Labcorp genomic profiling solutions to support your next oncology trial.
References
1 Mosele MF, Westphalen CB, Stenzinger A, et al. Ann Oncol. 2024;35(7):588-606. doi:10.1016/j.annonc.2024.04.005
2 Chakravarty D, Johnson A, Sklar J, et al. J Clin Oncol. 2022;40(11):1231-1258. doi:10.1200/JCO.21.02767
3 Pascual J, Attard G, Bidard FC, et al. Ann Oncol. 2022;33(8):750-768. doi:10.1016/j.annonc.2022.05.520
4 Dang DK, Park BH. J Clin Invest. 2022;132(12):e154941. doi:10.1172/JCI154941
5 Bayle A, Peyraud F, Belcaid L, et al. Ann Oncol. 2022;33(12):1328-1331. doi:10.1016/j.annonc.2022.08.089
6 Iams WT, Mackay M, Ben-Shachar R, et al. JAMA Netw Open. 2024;7(1):e2351700. Published 2024 Jan 2. doi:10.1001/jamanetworkopen.2023.51700
7 Use of Circulating Tumor DNA for Curative-Intent Solid Tumor Drug Development Guidance for Industry. U.S. Food and Drug Administration. 2024. https://www.fda.gov/media/183874/download
8 Godsey JH, Silvestro A, Barrett JC, et al. Clin Chem. 2020;66(9):1156-1166. doi:10.1093/clinchem/hvaa164