A young drug company has raised $125 million to get a medicine used for a rare kind of chronic pain approved in the U.S.
Officially launched on Tuesday, Ambros Therapeutics was co-founded by biotechnology entrepreneur and former presidential candidate Vivek Ramaswamy. The startup’s main asset is a new “bisphosphonate,” a type of drug that prevents bone loss. Research indicates these compounds also possess anti-inflammatory properties and can act as pain relievers.
Ambros’ medicine, called neridronate, was discovered, developed, and commercialized by another company, Abiogen Pharma, for the Italian market. There, it’s already approved to treat a couple of bone diseases as well as an uncommon disorder known as “complex regional pain syndrome Type I.”
In that latter condition, patients who’ve undergone surgery or suffered an injury, such as a broken bone or sprain, subsequently experience a disproportionate amount of pain from the initial trauma. The pain is persistent, and can feel like extreme burning, throbbing, stabbing or squeezing.
Ambros bought from Abiogen exclusive rights to neridronate in North America. Now, with its new Series A funding, the startup aims to begin in the first three months of next year a late-stage study that could serve as the foundation for an approval filing with the Food and Drug Administration.
In addition to financing that study, the money will be used for “related regulatory preparations and pre-commercial activities,” Ambros said in a statement. The FDA has already granted neridronate a few designations that could speed its regulatory review and give it commercial benefits. Ambros said an estimated 65,000 new cases of CRPS-1 occur annually in the U.S.
RA Capital Management and an arm of Patient Square Capital co-led the round. They were joined by Abiogen Pharma and more than half a dozen other investors.
"Neridronate is one of the few late-stage programs with a well-established mechanism, extensive real-world experience, and the potential to meaningfully change the trajectory of CRPS-1," said Matthew Hammond, an RA Capital partner, in that statement.
Ambros furthers a tear of investments from RA Capital, which, according to data compiled by BioPharma Dive, was the most prolific venture capital firm in biotech over the past four years. Since 2022, RA Capital has participated in 85 funding rounds, including more than a dozen this year.
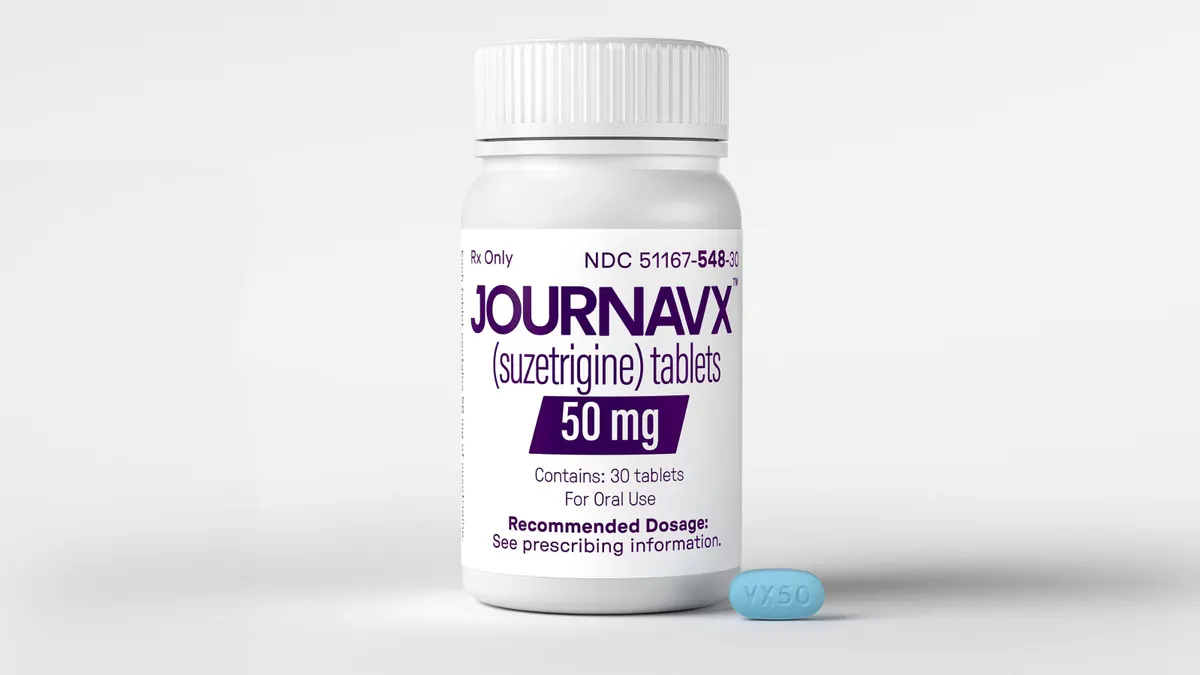
Ambros’ haul also comes at a time when pain research — long considered too risky a proposition for most life sciences backers — is drawing more interest, in good part because of the recent approval of a non-opioid pain medication from Vertex Pharmaceuticals.
Last December, for instance, the small biotech SiteOne Therapeutics raised $100 million to support its mission of creating new, non-addictive pain drugs. Novo Holdings, the controlling stakeholder of Ozempic-maker Novo Nordisk, led the round. Ken Harrison, a senior partner at Novo Holdings, said a main reason the firm decided to invest was that Vertex had set a precedent that these types of drugs can be successfully developed and brought to market.