Dive Brief:
- A drug combination involving Bristol Myers Squibb’s experimental medicine iberdomide met one of its main goals in a Phase 3 clinical trial, helping to eliminate signs of multiple myeloma in significantly more enrollees than a standard regimen, the company said Tuesday.
- The data are from an interim analysis of a study that will continue so trial investigators can measure other goals like an impact on disease progression and survival. Bristol Myers will submit the results to health regulators, although executives have previously said an approval would probably only come if iberdomide meets its other objectives.
- Iberdomide is one of three protein-degrading drugs Bristol Myers is positioning as successors to blockbuster blood cancer drugs like Revlimid and Pomalyst, which it acquired through its merger with Celgene. Many of the products in its large portfolio of cancer drugs have either plateaued or are in decline.
Dive Insight:
Called Excaliber-RRMM, the study Bristol Myers discussed Tuesday tested iberdomide alongside two other drugs, Darzalex and the steroid dexamethasone, and compared that regimen to a widely used treatment combination: Darzalex, dexamethasone and another medicine called Velcade. The trial enrolled people whose multiple myeloma returned after, or didn’t respond to, an initial treatment.
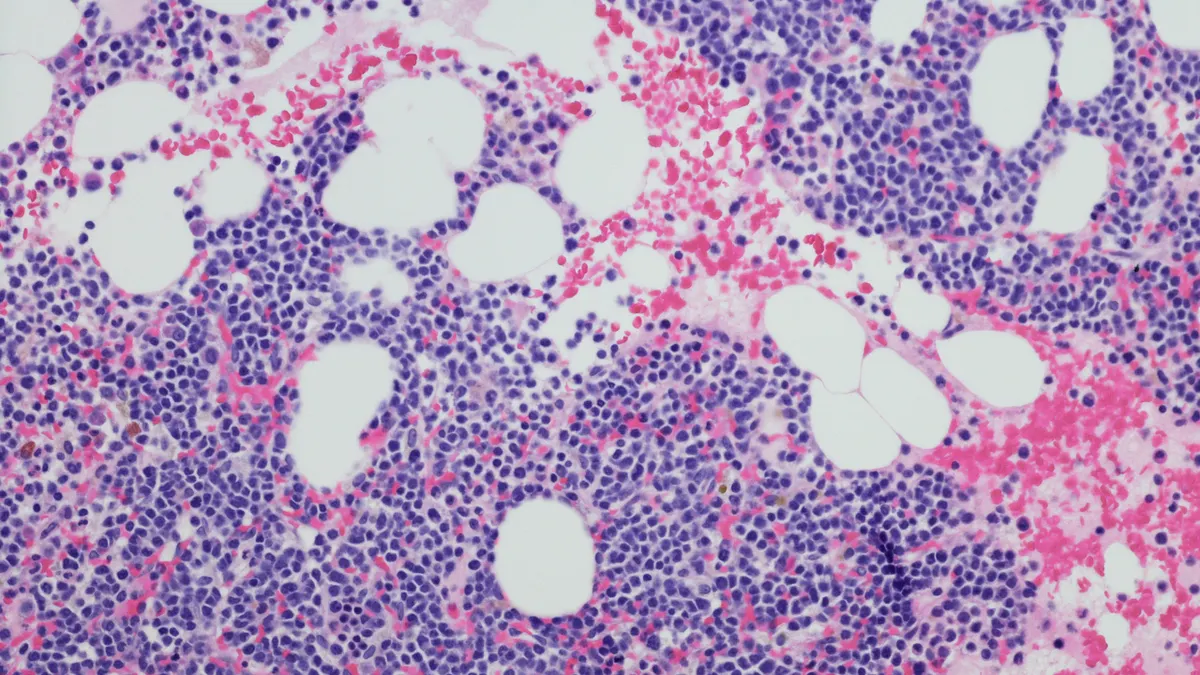
One of the study’s top goals was for iberdomide to be associated with an improvement in the rates of people who are “minimal residual disease negative,” or have a very low number of malignant cells in the bone marrow following treatment. Though Bristol Myers didn’t provide specifics, it said its treatment met that mark.
Still, iberdomide has more to prove. A second endpoint, progression-free survival, is also considered “co-primary” under the trial’s statistical analysis plan, meaning the study wouldn’t be successful unless it helps keep patients’ myeloma in check. Its ability to extend survival, a key secondary goal, will also be closely watched.
“We would have to look at the totality of the data, that other endpoints are moving in the right direction, because the supportive evidence would be needed from a regulatory perspective,” said Samit Hirawat, Bristol Myers’ then-chief medical officer, when asked about iberdomide’s approval prospects during a July conference call.
Drugs like iberdomide work by tagging certain proteins essential for cell survival for destruction by the body’s own regular disposal mechanisms. Bristol Myers sees them as next-generation versions of Revlimid, which hit nearly $13 billion in sales at its peak in 2021, and Pomalyst, which recorded sales of $3.5 billion in 2024.
Consensus estimates have iberdomide generating around $1.3 billion annual sales in 2035, Leerink Partners analyst David Risinger wrote in a July note to clients. “If the iberdomide data are promising, we see substantial upside” to that consensus, he wrote.