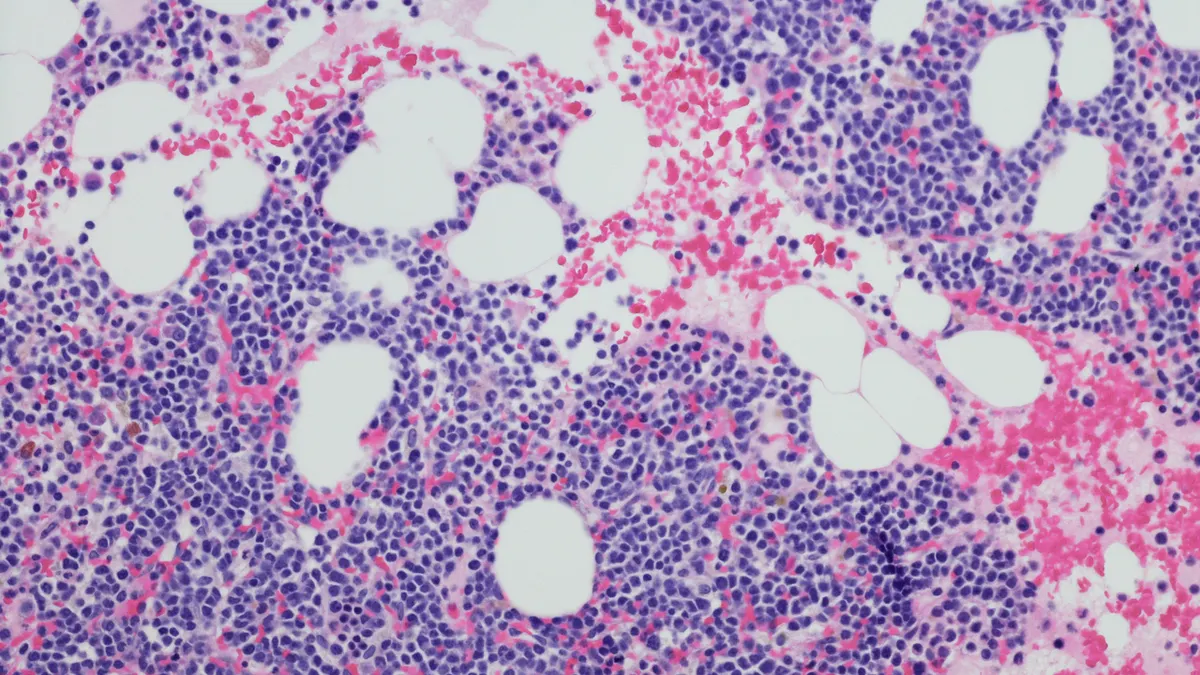
ORLANDO – A regimen involving Johnson & Johnson’s dual-acting drug Tecvayli could be curative when used early in the disease course of people with multiple myeloma, according to data disclosed Tuesday.
Released at the annual meeting of the American Society of Hematology in Orlando, the results come from a trial called MajesTEC-3. J&J in October claimed early success for the study, which evaluated Tecvayli alongside another J&J drug called Darzalex, against Darzalex and a standard combination in people whose disease had advanced after one to three treatment lines. But it didn’t provide specific details, saving them for a spotlighted presentation at ASH on Tuesday.
According to those results, the Tecvayli-Darzalex combination cut the relative risk of disease progression or death by 83% when compared to Darzalex and other therapies. Progression was also uncommon for treatment recipients who went six months without relapsing. According to J&J, 90% of those enrollees were still progression-free three years after the study’s start, leading researchers to suggest the combination could have curative potential.
“The efficacy is truly remarkable with this combination,” said Surbhi Sidana, an associate medical professor at Stanford University and a trial investigator. “We can see a light at the end of our tunnel with all of these therapies for our patients, having maybe a functional cure in the future.”
Tecvayli is one of four so-called bispecific antibodies available for multiple myeloma. All are cleared under “conditional” approvals, though, and only available for patients who’ve previously received at least four lines of treatment.
The MajesTEC-3 results show the potentially dramatic benefits of moving these drugs into earlier lines of care and combining them with other treatments, said one expert.
“What it does is prove two things. One is clearly that when you give bispecific antibodies earlier in the disease course they work better,” said Amrita Krishnan, executive medical director for hematology at City of Hope Orange County, in an interview. The other is that Darzalex “further augments the efficacy of [Tecvayli], and one would assume the same for other bispecifics,” added Krishnan, who wasn’t involved with the trial.
If approved for earlier use, Tecvayli would provide a more convenient, and potentially more broadly used alternative to the CAR-T cancer cell therapy Carvykti, she said. Carvykti, with J&J also markets, is made from a patient’s own cells. It is administered most often in a limited number of highly specialized cancer centers and less so in the community oncology practices that treat many patients.
“There are patients at early relapse who we do not want to [give] CAR-T, and a preference would be to give them a bispecific antibody,” she said. But bispecifics have only been available to patients much later on in their disease course, so “we’ve had to wait to be able to do that.”
Widening use of bispecifics in multiple myeloma has been one of J&J’s major objectives, said Imran Khan, its vice president of hematology medical affairs. “Up to 50% of patients are in the community. We want to ensure that every single patient has the opportunity to get the option that is best for them where they are in their treatment journey,” he said.
But broader use could bring new safety considerations, too. The presentation showed that 13 people in the Tecvayli-Darzalex group died from infections, and treatment recipients were more likely to contract COVID-19 and related pneumonia, as well as upper respiratory tract infections and other viral conditions. By comparison, patients who receive CAR-T therapies are closely monitored and even kept in hospitals to quickly receive care for side effects like infections.
MajesTEC-3 trial investigators changed medication protocols during the study to allow for immunoglobulin replacement therapy and antimicrobial prevention.
“Infection risk can be mitigated, but still, patients do get some infections,” Sidana said. “We have to be very, very vigilant, especially as we transition these drugs to the community, where doctors are treating many different diseases and they may not be so on top of things.”
J&J enrolled 587 people and randomized roughly half to the Tecvayli-Darzalex combination, with the rest receiving Darzalex, a commonly used steroid and either Velcade or Pomalyst. The study’s main objective was improving progression-free survival. Response rates, overall survival and achieving undetectable disease were considered secondary endpoints.
The Tecvayli-Darzalex combination induced a complete response, meaning patients had no trace of disease, in 82% of people who received it, compared with 32% of people who got the alternative combinations. The combination also reduced the relative risk of death by 54%.
Additionally, 83% percent of people who got Tecvayli were still alive three years after entering the trial, compared with 65% of people who got the other regimens.
Editor's note: This story has been corrected to reflect the use of Carvykti in community oncology practices.