Dive Brief:
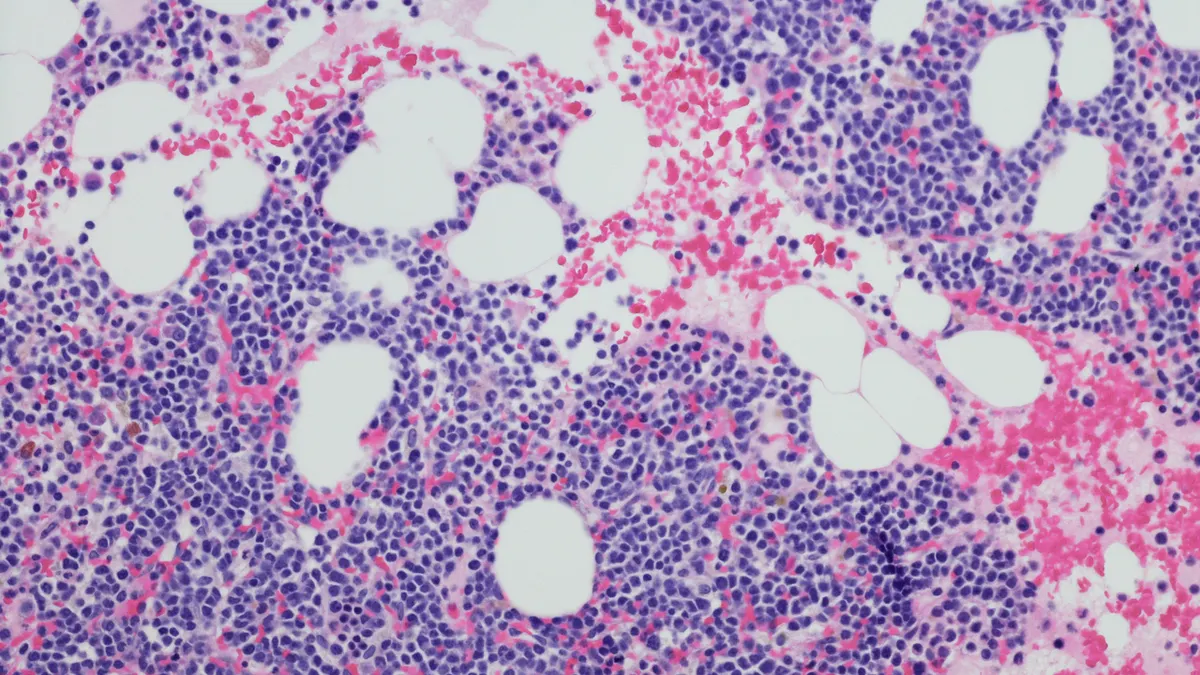
- Johnson & Johnson’s Tecvayli helped people with multiple myeloma live longer than those who’d received standard drug combinations in a Phase 3 trial, the company said Wednesday.
- J&J enrolled who’d relapsed after receiving frontline therapies and administered either Tecvayli — a dual-targeting antibody drug — or widely used regimens involving medicines like Velcade and Kyprolis. Trial enrollees who got Tecvayli were 71% less likely than those in the comparator group to die or have their disease progress during the trial, J&J said.
- This trial builds on evidence supporting early use of Tecvayli in multiple myeloma. At a medical meeting last year, J&J presented results showing a combination of Tecvayli and another drug it sells called Darzalex might be curative when administered early in a patient’s disease course.
Dive Insight:
Launched in 2022, Tecvayli was cleared under a conditional, “accelerated” approval and can only be used after four previous treatment lines. But J&J is working to make it available earlier as part of a broader push to build a dominant franchise in multiple myeloma. That business also includes Darzalex, the cell therapy Carvykti and another dual-acting antibody drug called Talvey.
The case J&J is making has already caught the attention of U.S. regulators. Only days after J&J detailed results from the Tecvayli-Darzalex trial, the Food and Drug Administration proactively reached out and awarded it one of the agency’s “national priority” regulatory fast passes.
The results of this latest trial to read out, called MajesTEC-9, will help, too. J&J enrolled 614 people whose disease had gotten worse after initial treatment with a Darzalex or Revlimid-based regimen, and randomized them to receive either Tecvayli or the alternative treatments. Tecvayli’s main goal was to improve progression-free survival.
Extending survival was one of several secondary objectives, and Tecvayli succeeded on that measure as well. J&J said treatment helped reduce the risk of death by 40% and that trial monitors detected a benefit after their first data check, prompting them to unblind the study.
The positive results from the two studies “help establish Tecvayli as an essential therapy for patients as early as first relapse," said Roberto Mina, an associate professor at Emory University, in a statement.
J&J said it will discuss the results of the trial with the FDA and other regulatory agencies, and present them at a future medical meeting.
Tecvayli, which J&J launched with a list price of about $39,000 a month, helps immune cells attack and destroy tumor cells by binding simultaneously to proteins found on both.