Each year, a small number of babies are born mostly, if not fully, deaf because one of their genes isn’t working.
The gene normally makes a protein that the hairs in our inner ears need to relay sound signals to the brain. Without that protein, people with this rare form of hearing loss often rely on cochlear implants for their entire lives.
But in the near future, genetic medicine may offer another option. On Sunday, fresh results from a small clinical trial showed that, among a dozen children given a gene therapy from Regeneron Pharmaceuticals, most are now hearing well enough to not need help from implants.
Encouraged by those results, Regeneron plans to submit an approval filing to the Food and Drug Administration by the end of the year.
“To have a therapy that gets kids back to essentially normal hearing in many cases, it's game changing for all of us in the field. It’s just super exciting,” said Lawrence Lustig, a trial investigator, hearing specialist and department chair at Columbia University Medical Center.
Hearing loss is often categorized by the softest sound level a person can register. On one end of the scale is normal, then mild, moderate, severe, and finally “profound,” or the most impaired. Regeneron last presented early, positive findings from its study in February, at which point researchers had collected six months of follow-up data on five children, three of whom were hearing at normal or near-normal levels.
Six-month data is now available for 12 participants. Of them, nine hit the study’s main goal by demonstrating their hearing was in the moderate to normal range at that time point. One of the remaining three had no improvement, while the two others had “positive changes.” Lustig noted that one of those latter children did end up in the moderate class almost a year after treatment.
The trial enrolled children of varying ages. The oldest could probably drive a car, while the youngest, at 10 months, was perhaps just learning to walk. In a paper published in The New England Journal of Medicine on Sunday, study investigators wrote how Regeneron’s therapy appeared beneficial across this group — “contrary to prevailing views” it might only be effective when given early in life.
The trial also assessed different configurations of ear treatments. Three children, for example, got the therapy in both ears. Everyone else only got it in just one. Four entered the study already having a cochlear implant in one ear, whereas some got the devices as part of the experiment.
The first trial participant, the 10-month old, was in that latter group. Her parents noticed changes within a few weeks post-treatment. She was reacting to hand claps and her name, and became more aware of speech and whispers. By the 24-week mark, her hearing had improved to normal levels, the study authors wrote.
At 72 weeks, researchers briefly turned off her implant to pinpoint how the therapy-treated ear was functioning. They found her speech perception had continued to improve. On a formal test, she identified with complete accuracy a series of two-syllable words like “mommy” and “cookies” without requiring visual cues. Later, at 96 weeks, she scored 70% on a single-syllable word test.
Another, 28-month-old participant got Regeneron’s therapy in one ear and no treatment in the other. Nearly a year later, her parents reported “spectacular” progress. They said their daughter could now respond to distant sounds and understand speech in noisy environments. She could also count to 10 and use more than 100 words — what they described as “unimaginable” improvements, according to the NEJM paper.
The therapy, code-named DB-OTO, is constructed with tiny, disarmed viruses, which deliver functional copies of that malfunctioning gene directly into the inner ear.
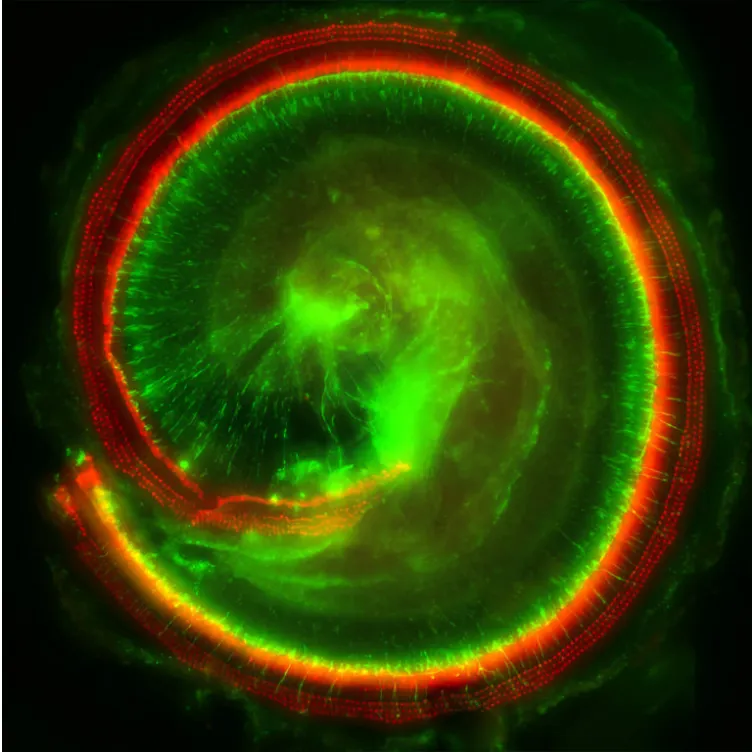
It’s an advantageous target, because people can develop antibodies that attack these viruses and, in turn, nullify the whole gene therapy. But a natural barrier separates the cochlea from the bloodstream, where most antibodies are on patrol. Indeed, 10 participants tested positive for neutralizing antibodies at the start of Regeneron’s study, yet these proteins didn’t appear to interfere with the gene therapy’s effectiveness.
DB-OTO is administered via the same surgical approach used for cochlear implants — a very calculated design feature. Jonathan Whitton, the global program head for Regeneron’s hearing research, said one key objective was to create a product surgeons would immediately feel comfortable with and know how to deliver.
So far, DB-OTO looks generally safe. Researchers have documented 67 so-called adverse events, including 17 deemed related to delivery procedures. Almost all were minor, short-lived and “the kind of things you would see in any sort of ear-related surgery,” like pain or nausea, according to Lustig.
There were, however, two serious events. One participant experienced instability while walking. Another developed a severe bacterial infection in an ear that got a cochlear implant but not DB-OTO. Both resolved without causing additional health problems.
The trial results naturally raise questions, like why some patients responded better than others.
And, as is the case with most gene therapies, researchers aren’t sure exactly how long the effects of DB-OTO last.
“This is the first time this has ever been done. What's going to happen five, 10 years down the road? Will we have to re-dose? These are all big questions we're going to have to answer,” Lustig said. “But at least even in our long-term kids out over a year, the results are holding and, if anything, improving.”
Should DB-OTO reach the market, insurance companies will surely take a close look at the durability of what’s bound to be an expensive medicine. In the U.S., list prices on gene therapies stretch from hundreds of thousands of dollars to several million.
The Regeneron team, though, views the apparent benefits as worth the likely high cost.
“What we're talking about, really, is: what's the value of hearing?” said Whitton. “My expectation is most people see massive value, having hearing connect you to your environment, to others, to your families.”
Whitton previously served as the head of clinical research at Decibel Therapeutics, the original developer of DB-OTO that Regeneron bought for $109 million in 2023. One of only a few all-out acquisitions in Regeneron’s almost 40-year history, Decibel proved to be a harbinger of the company’s growing interest in genetic medicine.
In the months and years since, Regeneron has expanded a gene editing pact with Intellia Therapeutics, purchased a slate of cell therapies from 2Seventy Bio, and paid $100 million for access to Mammoth Biosciences’ CRISPR technology. The company also attempted to snag some of 23andMe’s assets as the DNA testing firm went through bankruptcy proceedings.
Such deals place Regeneron at the center of a field that is both cutting-edge, but also one that investors decidedly cooled on over the past few years.
Even with supportive clinical data, Regeneron may well face challenges selling a product like DB-OTO.
David Risinger, an analyst at the investment firm Leerink Partners, laid out some of the potential obstacles in a note to clients last year. He wrote that around 20,000 people in the U.S. and European Union have hearing loss caused by the mutations addressed by Regeneron’s therapy. Yet only those who don’t already have cochlear implants in both ears would be eligible for DB-OTO.
Additionally, at least three other companies are developing their own gene therapies for this condition, making for a “crowded competitive landscape.” One of those companies, Akouos, was acquired by Eli Lilly in a deal that, at the high end, could be worth roughly $610 million.