Predicting Clinical Response to PD-1 Checkpoint Inhibition
The traditional course of drug development in oncology involves a complex array of standardized preclinical tests, predominantly focused on safety and toxicity, and the subsequent progression to clinical testing in human subjects. Clinical development of any drug typically comprises a phased series of studies and/or trials, designed to progress the drug through key regulatory milestones, ultimately leading towards approval of the drug in a specific indication(s) (Figure 1).¹’² Data characterizing the safety profile and clinical activity are systematically generated within each defined phase, with early-phase trials often also incorporating dose determinations, pharmacokinetic/pharmacodynamic (PK/PD) and absorption, distribution, metabolism and excretion (ADME) endpoints.
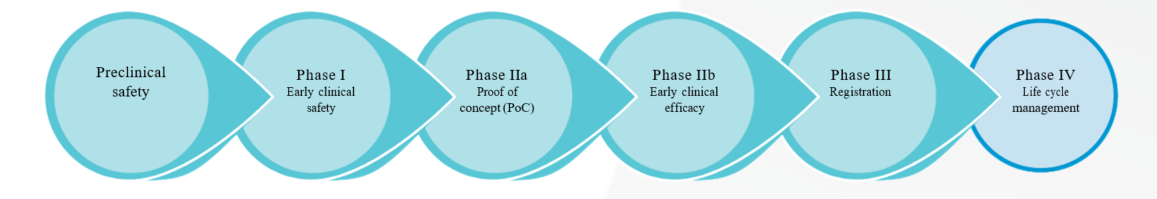
Unique to oncology, this historically linear approach to clinical testing begins with exposing a small population of patients to the drug, rather than healthy volunteers.1 The appropriate design and execution of early-phase clinical trials is of paramount importance to achieving PoC and establishing preliminary dosing schedules, and the data obtained should be robust and meaningful, thus enabling progression to testing in larger patient populations.
This white paper considers the recent paradigm shifts in oncology drug development and explores the current requirements of early-phase oncology trials. By remaining attuned to the dynamic nature of oncology drug development, time, cost and risk can all be efficiently minimized while evaluating and successfully managing safety and ethical considerations during the early clinical development journey.
Paradigm Shifts in Oncology Drug Development
There has been a significant increase in our knowledge of the molecular underpinnings of cancer in recent years, which has enabled tremendous advances across the oncology treatment landscape. The era of precision medicine is now upon us, and there has been a surge in the development of targeted therapies, including novel small-molecule and immunotherapeutic treatments, the impact of which may be further enhanced by the concomitant identification and validation of associated biomarkers.3 The need for new trial designs that more efficiently and effectively determine whether there is a link between biological activity and clinical activity has never been greater.
Trial designs accommodating the advances in oncology include basket trials, where drugs are tested across multiple disease subtypes or histologies, and adaptive trials, where flexible study designs are reactive to incoming data and can be changed based on predefined decision rules. Adaptive trials may evolve over time to test new agents, and drugs within a study may be removed or replaced where limited activity or excessive toxicity has been identified. There is also a steady increase in the number of first-in-human (FIH) trials that incorporate large expansion cohorts, enabling expedited development of new oncology drugs³.
The oncology treatment paradigm continues to shift as further advancements are made with regards to technology and our own understanding, and the distinct phases of development become increasingly blurred.² Flexibility on the part of drug developers and their collaborators is required continuously, and a deep understanding of how to react in the current competitive landscape is vital to success.
Adapting to the Requirements of Oncology Early Clinical Development
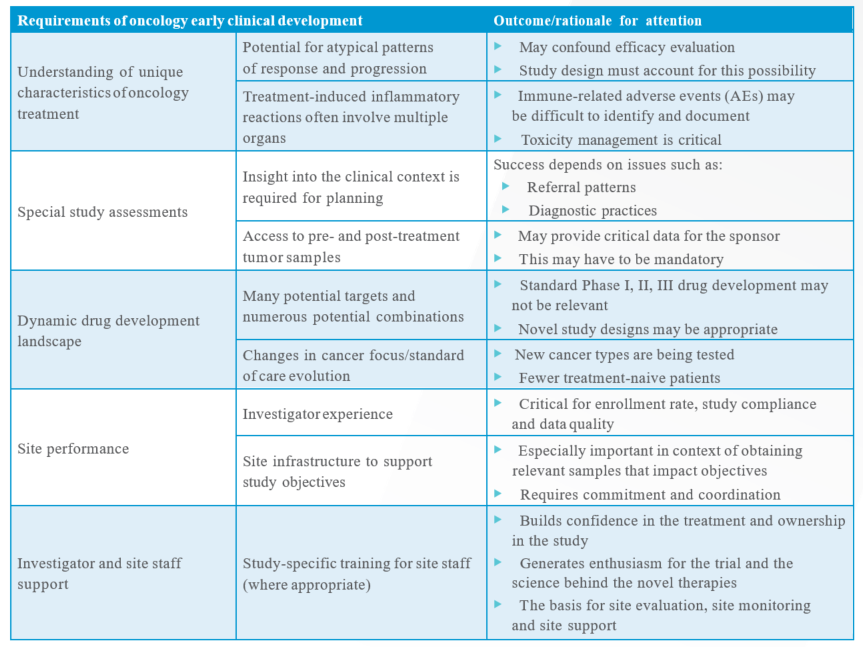
The dynamic development landscape is just one factor that requires adaptation on the part of the oncology early-phase trial sponsor(s). Several crucial considerations when designing and implementing an early-phase oncology trial are summarized in Table 1.
Flexible Partnerships Through Early Development and Beyond
All pharmaceutical and biotech companies, large or small, face the same core issues when approaching oncology early-phase clinical trials. These include identifying challenges early to ensure time and cost are not impacted, and aligning a nonclinical plan with clinical endpoints to expedite the path to FIH studies.
Covance is adept at listening to and understanding the individual needs of each client, often operating as a ‘CRO within a CRO’ for smaller clients, using teams comprised of experts in early-phase trial execution. Nurturing a long-lasting partnership that provides continuity in the development process can be key to succeeding in today’s developmental paradigm flux. Whether a hands-on approach is required or a more distant partnership is preferred, Covance provides the flexibility, proactivity and dedication needed to take molecules successfully from the early stages of development to proof of concept and into late-stage clinical trials.
References
- Dhingra, K. (2015). Oncology 2020: A drug development and approval paradigm. Annals of Oncology, 26(11), 2347-2350.
- Prowell, T. M., et al. (2016). Seamless Oncology Drug Development. The New England Journal of Medicine, 374(21), 2001-2003.
- Emens, L. A., et al. (2016). Cancer immunotherapy trials: leading a paradigm shift in drug development. Journal of Immunotherapy of Cancer, 4, 42
Covance's Expertise in Oncology Early Clinical Development
Expert teams comprising nonclinical, clinical, regulatory and health economic advisors within Covance share the common goal of accelerating a client’s molecules through critical milestones towards the next stages of development. These teams are supported by project managers specializing in oncology, and the integrated central laboratory services, found at five sites strategically placed worldwide.
Within the last five years, Covance has conducted 270 oncology studies involving more than 8,500 sites in 60 countries, enrolling over 40,858 patients globally. Of these, 186 were Phase I studies, which employed 1,070 sites and enrolled 7,635 patients.
Covance prides itself in providing clients with continuity in oncology clinical development via a lasting partnership that begins at early clinical development and progresses through the development phases. We build strong and successful relationships to help clients drive their molecules efficiently from PoC all the way through to approval and launch.