Drug Pricing: Page 4
-
Biogen, Sage set price of postpartum depression pill at $15,900
The price is below what some analysts had predicted and significantly less than $34,000 Sage initially set for its earlier postpartum infusion, Zulresso.
By Ned Pagliarulo • Nov. 7, 2023 -
PBMs, PhRMA trade blame over drug costs in House hearing
Pharmacy benefit manager lobby PCMA and drugmaker lobby PhRMA pointed fingers over problems in the prescription supply chain during the House committee's second hearing on the PBM industry.
By Rebecca Pifer Parduhn • Sept. 19, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Elizabeth Regan/BioPharma Dive
Elizabeth Regan/BioPharma Dive Trendline
TrendlineDrug pricing
President Trump’s efforts to install a most-favored nation pricing policy present new risks for an industry already adjusting to Medicare price negotiations.
By BioPharma Dive staff -
Astellas withdraws lawsuit challenging Medicare drug price program
The drugmaker said it still believes the U.S. government’s price negotiation powers are unconstitutional, but is pulling back a lawsuit it filed in July.
By Ned Pagliarulo • Sept. 7, 2023 -
Medicare named the first 10 drugs up for negotiation. Now what?
The list of blockbuster cardiovascular, diabetes and cancer drugs gives the industry a window into how regulators are approaching price negotiations.
By Karissa Waddick • Aug. 30, 2023 -
Medicare names first 10 drugs for price negotiations
The Inflation Reduction Act gave the U.S. government new pricing powers, which CMS put into practice this August with its first slate of blockbuster targets.
By Ned Pagliarulo • Updated Aug. 29, 2023 -
AstraZeneca the latest pharma to challenge drug pricing law
The drugmaker's lawsuit follows similar legal challenges from Merck & Co., Bristol Myers Squibb and Johnson & Johnson, among others.
By Ned Pagliarulo • Aug. 25, 2023 -
CVS launches new venture in biosimilar drug experiment
The subsidiary, called Cordavis, will work directly with manufacturers to market or co-produce low-cost biologic drugs, starting with Novartis’ Humira copy.
By Jonathan Gardner • Aug. 24, 2023 -
Boehringer sues to block US drug price program
The pharma’s suit, which claims Medicare’s new power to negotiate certain drug prices is unconstitutional, comes days before the agency will reveal the first 10 medicines to be included under the plan.
By Ned Pagliarulo • Aug. 22, 2023 -
Blue Shield of California drops CVS Caremark in pharmacy benefit overhaul
BSCA has kicked CVS Caremark, the largest pharmacy benefit manager in the country, to the curb and is electing to carve out various pharmacy functions with companies like Amazon instead.
By Rebecca Pifer Parduhn • Updated Aug. 17, 2023 -
EQRx to sell to Revolution Medicines after failed bid to upend US drug pricing
The all-stock deal is a quiet end for the ambitious biotech, which was sold for its billion-dollar bank account after the FDA derailed its plans to develop low-cost cancer medicines.
By Ben Fidler • Aug. 1, 2023 -
GSK confident in RSV vaccine launch, but sets expectations for ‘steady build’
GSK expects its respiratory syncytial virus vaccine will be a multibillion dollar product. But at the beginning, it’s predicting a slower launch than for its fast-selling shingles shot.
By Delilah Alvarado • July 27, 2023 -
Patent thickets
Biosimilar makers split strategies in bid to take on top-selling Humira
In challenging AbbVie for share of a $19 billion drug market, competitors are testing whether high upfront discounts or behind-the-scenes rebates can win them an advantage.
By Jonathan Gardner • July 26, 2023 -
J&J joins pharma allies in challenging US drug pricing law
The pharma claimed in federal court that the Inflation Reduction Act, which threatens sales of its blood thinner Xarelto, amounts to “confiscation of constitutionally protected property.”
By Kristin Jensen • July 19, 2023 -
Pharma’s strike-from-all-sides attack on the IRA could end up at the Supreme Court
Industry lawsuits have taken a ‘multi-pronged’ approach to challenge the Inflation Reduction Act’s price negotiation program on constitutional grounds.
By Alexandra Pecci • July 6, 2023 -
Alvotech to raise cash after third FDA rejection for Humira biosimilar
The latest regulatory setback, tied to continuing manufacturing issues, ensures Alvotech and partner Teva won’t be able to launch their drug alongside a wave of emerging Humira copycats.
By Kristin Jensen • June 29, 2023 -
Sarepta prices Duchenne gene therapy at $3.2M
The cost makes Sarepta’s treatment among the most expensive medicines in the world. But company executives said they don’t expect significant insurer pushback.
By Ben Fidler • June 22, 2023 -
AbbVie, Coherus mend dispute over low-price Humira biosimilar
The biosimilar developer’s plans to set steep discounts on its copycat version of AbbVie’s Humira set off a legal back-and-forth between the companies.
By Kristin Jensen • June 15, 2023 -
Pharmaceutical companies search for IRA response as they brace for drug pricing talks
Lobbying group PhRMA does not expect any near-term changes to the IRA’s drug pricing provisions, but is attempting to lay groundwork for future changes.
By Christopher Newman • June 15, 2023 -
Medicare sets next tranche of drugs to face price hike penalties
CMS named 43 Part B drugs for which prices rose more than inflation, and which will have lower coinsurance rates next quarter as a result.
By Ned Pagliarulo • June 9, 2023 -
Merck sues to block Medicare negotiation of drug prices
The drugmaker’s lawsuit claims the new pricing powers granted by the Inflation Reduction Act are unconstitutional and “tantamount to extortion.”
By Christopher Newman • Updated June 6, 2023 -
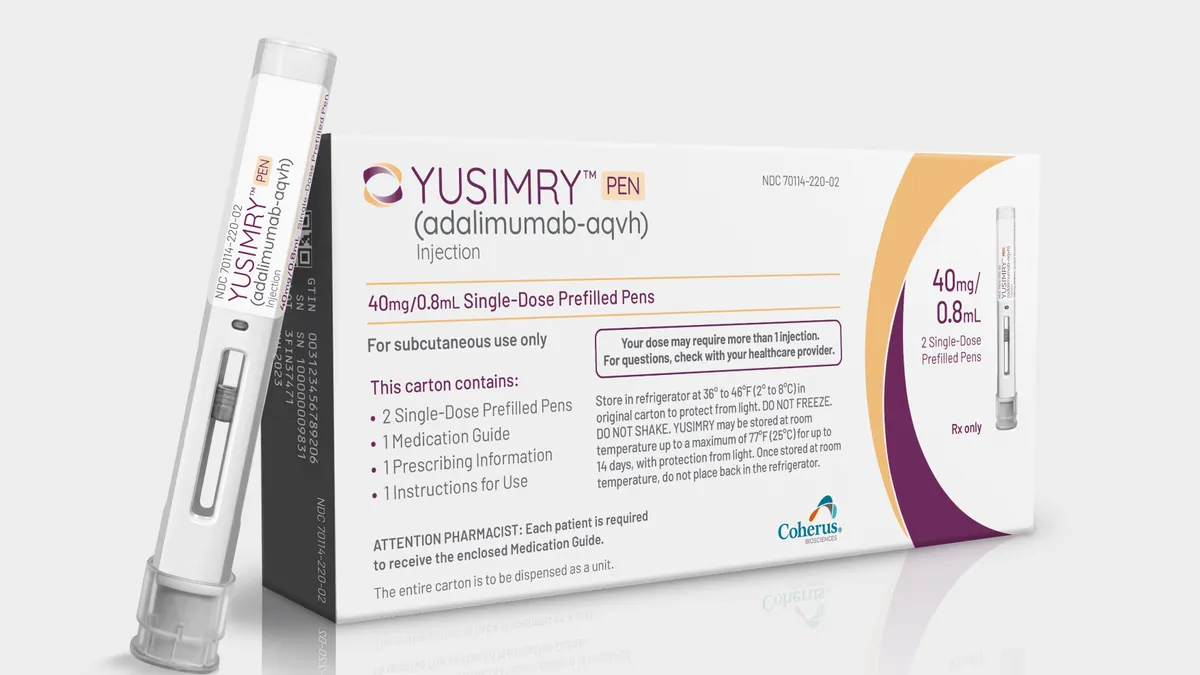
Coherus sets steep discount for Humira copycat, plans direct sales
The biotech will price its biosimilar Yusimry at 85% below Humira’s list price when it launches next month, and charge even less if bought through Mark Cuban’s pharmacy.
By Christopher Newman • June 1, 2023 -
House lawmakers, PBM lobby spar over committee hearing
At the latest congressional inquiry into pharmacy benefit managers, lawmakers argued the middlemen profit at the expense of patients and taxpayers.
By Rebecca Pifer Parduhn • May 25, 2023 -
AbbVie weathers first months of biosimilar challenge to top-selling Humira
While Humira sales declined, the drop was mostly due to price concessions AbbVie made to secure equal insurer access in the face of copycat competition.
By Ned Pagliarulo • April 28, 2023 -
CMS removes 7 drugs from list marked for price hike penalties
After revising its calculations, CMS removed Gilead’s cancer cell therapies Yescarta and Tecartus, as well as 5 other medicines, from the first set of drugs subject to rebates under a provision of the IRA.
By Christopher Newman • March 31, 2023 -
Ohio AG sues Cigna, Humana for alleged PBM price fixing
The state’s Attorney General David Yost called pharmacy benefit managers “modern gangsters,” claiming in a suit filed Monday they shared pricing information and drove up drug prices.
By Hailey Mensik • March 28, 2023