Dive Brief:
- A regimen pairing Johnson & Johnson’s dual-pronged multiple myeloma drug Tecvayli with an older medication, Darzalex, staved off disease progression and death better than Darzalex and a standard drug combination in a Phase 3 trial, the company said Thursday.
- According to J&J, a panel of independent trial monitors recommended halting the study early after the Tecvayli regimen met its objectives at an early data check. Researchers have been following trial volunteers for an average of about three years.
- The trial assessed the Tecvayli combination in people whose multiple myeloma had progressed after one to three prior treatment lines. Tecvayli is currently available to patients who’ve previously received at least four lines of care. That clearance, awarded in 2022, was an “accelerated” approval, which requires confirmation from a trial that demonstrates a survival benefit.
Dive Insight:
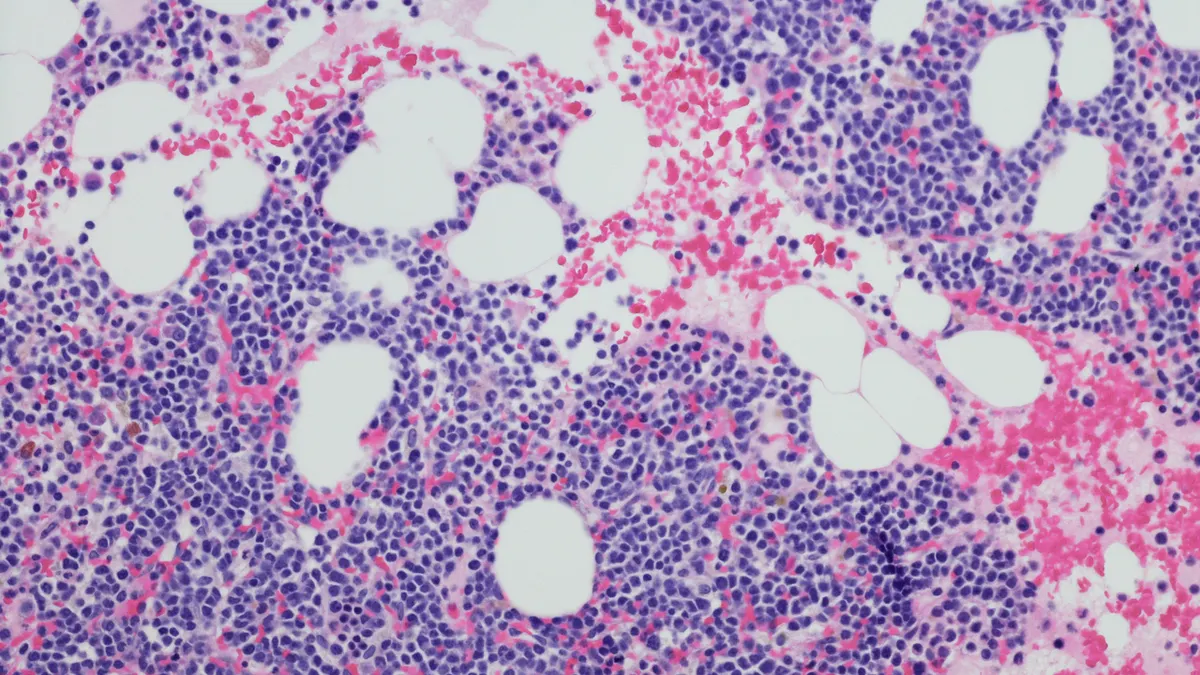
Tecvayli is one of many medications reshaping treatment of multiple myeloma, a persistent cancer in which the body produces dysfunctional versions of a type of white blood cell. It’s also among a new crop of drugs specially designed to target a protein called BCMA that’s frequently expressed on those diseased cells.
J&J has been heavily involved in those advances. The company already markets Darzalex, one of the world’s best-selling multiple myeloma medicines. But it also owns Tecvayli, a BCMA-targeting cell therapy called Carvykti, and another drug known as Talvey. Talvey and Tecvayli are “bispecific” antibodies that bind to targets on immune cells as well as cancerous ones. They’re seen as more convenient alternatives to personalized cell therapies like Carvykti.
Over the years, J&J has been moving its treatments into earlier treatment settings. Darzalex is already part of frontline care, and Carvykti last year became available to people whose disease has progressed following a single treatment line.
Tecvayli may be on a similar trajectory. The drug’s initial accelerated approval relegated it to later-line care. It also required J&J to follow up with confirmatory trials that measure survival rather than remission rates, necessitating further studies such as the trial that read out results on Thursday.
Called MajesTEC-3, the trial compared the Tecvayli-Darzalex regimen to a combination of Darzalex, the steroid dexamethasone and either Pomalyst or Velcade. The company didn’t disclose detailed results, but claimed the Tecvayli-based regimen was “statistically significant and superior” to its comparator on the study’s main measure of disease progression. A clear survival benefit was also observed at the trial’s first interim analysis, J&J said.
"We are confident this combination is poised to be a new standard of care option,” said Yusri Elsayed, the head of oncology at J&J’s innovative medicines division, in a statement.
Detailed data will be revealed at an upcoming medical meeting and shared with global health authorities.