Biotech: Page 18
-
Orca Bio to seek approval of T cell transplant after positive trial data
Results showed Orca’s treatment, a purified mix of donor-derived T cells and stem cells, was safer than standard transplant in treating certain blood cancers.
By Ned Pagliarulo • March 17, 2025 -
Pain drugs
Latigo raises $150M to get non-opioid pain drugs through key tests
The round, which included more than a dozen investment firms, should give the startup enough cash to get late-stage data for its lead candidate.
By Jacob Bell • March 17, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
Sponsored by 1nHealth
How biotech and pharma teams are reaching LPI ahead of schedule
With the right strategy, reaching LPI ahead of schedule isn’t a myth. Learn how some biotech and pharma teams are achieving the impossible.
By Katie Rodammer and Ryan Day • March 17, 2025 -
Makary advances to full senate vote; Acelyrin, Pliant adopt ‘poison pills’
President Trump’s nominee to run the FDA secured the Senate HELP Committee’s backing. Elsewhere, Sutro cut jobs and a cell and gene therapy pilot appeared to survive.
By BioPharma Dive staff • March 14, 2025 -
MeiraGTx spins Parkinson’s, obesity gene therapies into AI startup
The joint venture with generative AI firm Hologen hands MeiraGTx $200 million up front as well as other financial perks — a “transformative” deal, according to the company’s CEO.
By Jacob Bell • March 13, 2025 -
Obesity drugs
Roche broadens obesity drug plans with $1.65B Zealand deal
The deal gives the Swiss pharma access to an experimental amylin-targeting treatment it will test in combination with drugs it acquired from Carmot.
By Jonathan Gardner • March 12, 2025 -
A new obesity biotech launches; Gilead plans to quickly advance once-yearly PrEP for HIV
Harbour BioMed created Élancé Therapeutics to develop bispecific antibody drugs for weight loss. Elsewhere, Geron’s CEO is departing after 14 years.
By BioPharma Dive staff • March 12, 2025 -
Flagship startup raises $200M in pursuit of ‘scientific superintelligence’
Funded by investors such as Flagship and General Catalyst, the AI startup claims its platform will help scientists speed up the experimentation process.
By Gwendolyn Wu • March 12, 2025 -
Ono pays $280M to license Ionis rare disease drug
Ionis’ drug, called sapablursen, is currently being tested in a Phase 2 trial in people with polycythemia vera.
By Kristin Jensen • March 12, 2025 -
Viking inks CordenPharma deal to boost obesity drug supply
The biotech will commit $150 million to an alliance that hands it significant production capacity for a closely watched weight loss treatment.
By Jonathan Gardner • March 11, 2025 -
Arvinas gets positive breast cancer data, but finds differentiation a hard sell
Trial results show vepdegestrant, a protein-degrading drug Arvinas is developing with Pfizer, improved on fulvestrant in a subgroup of breast cancer patients, but not the overall study population.
By Ned Pagliarulo • March 11, 2025 -
Gene editing
Beam base editing therapy gets ‘proof of concept’ in rare lung disease
While initial study results suggest Beam's technology can correct alpha-1 antitrypsin deficiency's genetic roots, shares fell by double digits.
By Ned Pagliarulo • March 10, 2025 -
Roche launches new Boston center; NIH centralizes peer review
The Swiss pharma will base cardiometabolic drug research at the new Allston site. Elsewhere, AskBio, Bayer’s gene therapy subsidiary, advanced an experimental treatment for limb-girdle muscular dystrophy.
By BioPharma Dive staff • March 7, 2025 -
Nimbus swaps CEOs and turns to new leader for ‘next chapter’
CBO Abbas Kazimi will replace longtime CEO Jeb Keiper as part of a planned leadership transition at the unorthodox drug startup.
By Ben Fidler • March 7, 2025 -
Pain drugs
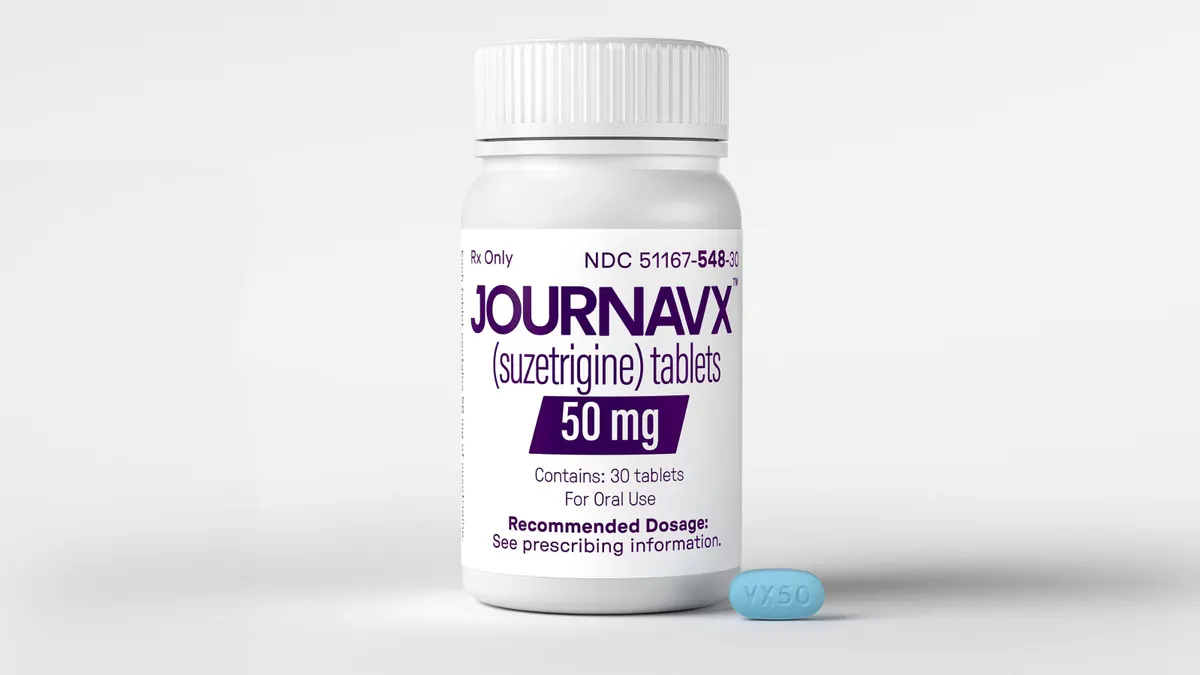
Vertex’s new pain drug gets first coverage nod from major insurer
Optum Rx, the pharmacy benefit manager owned by UnitedHealth Group, placed Journavx on some of its commercial formularies in an interim decision while a formal review continues.
By Jacob Bell • March 6, 2025 -
Trump administration
Federal judge extends block on Trump plan to limit NIH payouts
A District Court judge issued a nationwide preliminary injunction against the administration’s plan to cap so-called indirect costs associated with NIH grants — a policy with broad implications for the U.S. drug industry.
By Kristin Jensen • March 6, 2025 -
Amgen readies pivotal MariTide trials; Novo offers discounted Wegovy
Phase 3 studies for Amgen’s closely watched obesity drug were posted on a federal database. Elsewhere, the FDA cleared for testing a first-of-its-kind base editing therapy for Duchenne.
By BioPharma Dive staff • March 6, 2025 -
Sofinnova secures €1.2B for new biotech investments
The funding can support investment in as many as 60 new life sciences companies, said Sofinnova chairman Antoine Papiernik.
By Gwendolyn Wu • March 4, 2025 -
Neumora bids farewell to R&D head; Biohaven’s ‘one step forward, one step back’ data
Robert Lenz is leaving the brain drug developer to “pursue other interests.” Elsewhere, Atara is cutting half of its staff after shelving two cell therapy programs.
By BioPharma Dive staff • March 4, 2025 -
Deep Dive // State of Play
Blocking PD-1 and VEGF: The bispecific cancer drugs that could best Keytruda
Striking study results last year indicated a new type of medicine may improve on Merck’s immunotherapy, spurring a wave of research practically overnight.
By Ben Fidler • March 4, 2025 -
Startup launches
Biotech startup pulls in $187M to make ‘multi-payload’ ADCs
Callio Therapeutics is launching with an ADC technology and programs licensed from Singapore-based antibody drug developer Hummingbird Bioscience.
By Gwendolyn Wu • March 3, 2025 -

 Retrieved from National Cancer Institute on September 27, 2019
Retrieved from National Cancer Institute on September 27, 2019
BridgeBio oncology spinout to go public in blank-check merger
The deal comes amid an uptick in so-called SPAC deals, which had fallen out of favor due to poor returns and increased federal oversight.
By Ben Fidler • Feb. 28, 2025 -
Praxis hits trial setback; Bristol Myers lays off staff in New Jersey
The brain drug developer is continuing a trial despite a recommendation it be stopped for futility. Elsewhere, Eli Lilly struck a molecular glue deal and Zevra sold a priority review voucher.
By BioPharma Dive staff • Feb. 28, 2025 -
Gene editing
Vertex ends gene editing research pact with Verve
Verve offered few details on the early end of a collaboration the companies inked in 2022, saying only that Vertex cited “changing priorities.”
By Kristin Jensen • Feb. 27, 2025 -
AstraZeneca’s next breast cancer drug; Madrigal’s accelerating MASH launch
The British drugmaker claimed success in a first-line breast cancer study. Elsewhere, Rezdiffra sales continued to grow and a cancer biotech cut staff.
By BioPharma Dive staff • Feb. 26, 2025