FDA: Page 29
-
FDA delays decision on Bristol Myers cell therapy, putting Celgene deal payouts in jeopardy
Travel restrictions have made it difficult for the regulator to inspect a plant used to make the treatment, meaning the drugmaker could miss a deadline critical to former Celgene shareholders.
By Jonathan Gardner • Nov. 17, 2020 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

FDA unexpectedly rejects Alkermes schizophrenia drug, citing manufacturing concerns
Though Alkermes believes it can quickly resolve the issues cited by the agency, the complete response letter nonetheless delays approval of a drug that's critical to the company's hopes of a turnaround.
By Kristin Jensen • Nov. 17, 2020 -
 National Institute of Allergy and Infectious Disease. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Disease. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
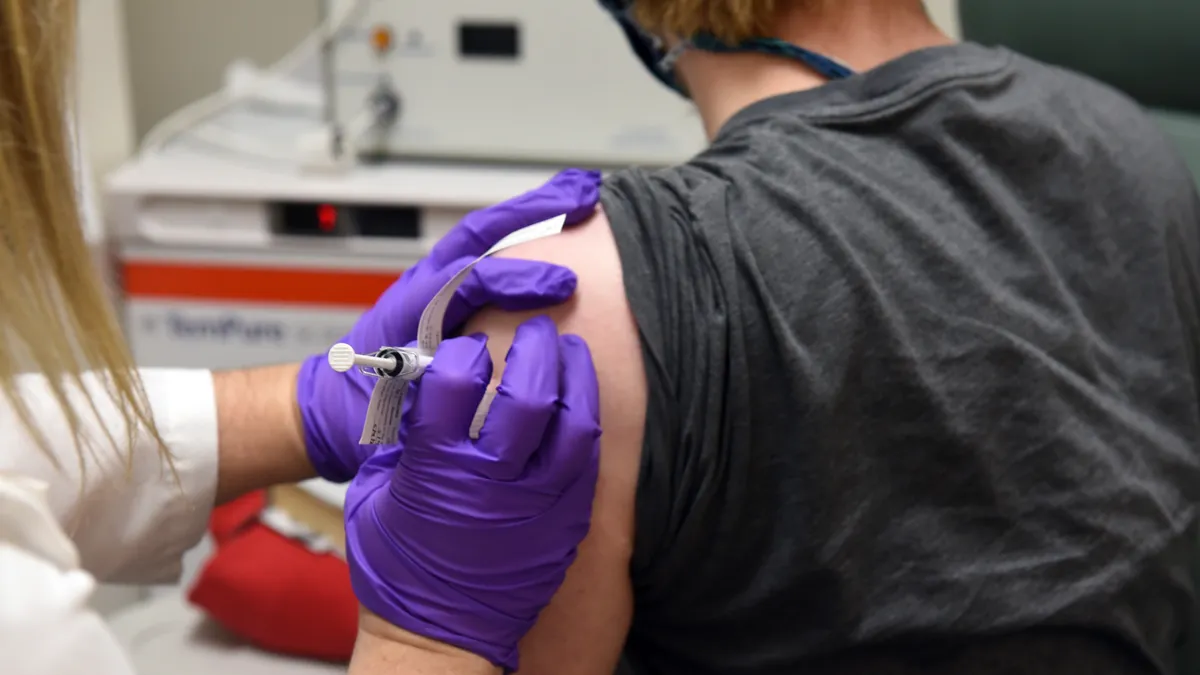
Eli Lilly wins FDA emergency clearance for COVID-19 antibody drug
The regulator approved Lilly's synthetic antibody treatment for emergency use, but short supplies and logistical challenges could limit its initial impact.
By Ben Fidler • Nov. 9, 2020 -
5 takeaways from the FDA's high-stakes meeting for Biogen's Alzheimer's drug
The expert panel's decidedly negative vote on aducanumab raised questions about the drug's future, as well as the credibility of the FDA.
By Jacob Bell , Ned Pagliarulo , Ben Fidler • Nov. 9, 2020 -
FDA advisers vote against Biogen's Alzheimer's drug, leaving its future in doubt
In a tense meeting, a group of experts found Biogen and the FDA's case for aducanumab unpersuasive, voting overwhelmingly against the drug.
By Jacob Bell , Jonathan Gardner , Ned Pagliarulo • Updated Nov. 7, 2020 -
BioMarin, stung by FDA rejection, pressed on path forward for 2 key drugs
Roctavian, the biotech's hemophilia gene therapy, was turned down by the FDA in August, while an important rare disease drug is now under review.
By Ned Pagliarulo • Nov. 6, 2020 -
Who would get Regeneron's COVID-19 antibody treatment?
The drug, now being considered for emergency clearance by the FDA, seems to work best in patients whose immune systems haven't responded to infection. Fast antibody testing, however, is spotty.
By Jonathan Gardner • Nov. 5, 2020 -
Biogen surges as FDA reviewers appear supportive of Alzheimer's drug approval
Documents made public ahead of a key advisory meeting Friday revealed an FDA more willing than expected to accept Biogen's controversial case for approval of aducanumab.
By Ned Pagliarulo , Jacob Bell • Updated Nov. 4, 2020 -
5 takeaways from the FDA's closely watched coronavirus vaccine meeting
Experts convened by the regulators debated how much data would warrant an approval, while pressing government officials on their distribution plans.
By Ned Pagliarulo , Jonathan Gardner • Oct. 24, 2020 -
AstraZeneca, J&J cleared to restart major coronavirus vaccine studies
The U.S. trial of AstraZeneca's shot has been paused over safety concerns since early September, while J&J's was halted in mid-October.
By Ned Pagliarulo • Updated Oct. 24, 2020 -
At key meeting, FDA advisors debate if early coronavirus vaccine approvals may compromise trials
An emergency clearance is expected for the first vaccine proven effective. But early availability of a shot could jeopardize ongoing studies, and may make tests of other candidates more difficult.
By Ned Pagliarulo • Oct. 23, 2020 -
Gilead's Veklury becomes first FDA-approved drug for COVID-19
The U.S. regulator granted a full approval after allowing emergency use earlier this year. New results from an WHO study, however, raise questions about the drug's ultimate effectiveness.
By Ben Fidler • Updated Oct. 23, 2020 -
Lilly hires consultant to help fix issues at COVID-19 drug manufacturing plant
The New Jersey facility that ran afoul of FDA manufacturing standards is one of several Lilly is using to produce coronavirus antibody drugs.
By Kristin Jensen • Oct. 22, 2020 -
Q&A
Coronavirus vaccine trial leader Larry Corey on the tough FDA, policy decisions to come
The co-leader of an NIH network of coronavirus prevention studies spoke with BioPharma Dive about the FDA's big vaccine meeting this week and what will come next.
By Ben Fidler • Oct. 20, 2020 -
Pfizer won't seek FDA clearance of coronavirus vaccine until mid-November, CEO says
An unusual letter from Pfizer chief Albert Bourla confirms that the drugmaker won't file for an approval of its experimental shot, assuming positive results, before the U.S. presidential election.
By Ben Fidler • Oct. 16, 2020 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

5 questions ahead of this week's FDA meeting on coronavirus vaccines
Normally staid affairs, this Thursday's advisory committee meeting will be closely watched and could set expectations for how the FDA will approach any future vaccine approval.
By Jonathan Gardner • Oct. 16, 2020 -
FDA approves Regeneron antibody drug as first Ebola virus treatment
Research for the drug, now cleared as Inmazeb, helped Regeneron jumpstart its fast development of an antibody therapy for COVID-19.
By Ned Pagliarulo • Oct. 14, 2020 -
Alkermes' comeback bid boosted by FDA panel backing for schizophrenia drug
While the committee's votes in favor of ALKS 3831 make an approval more likely, the drug's effects on patients also taking opioids could limit its use.
By Ben Fidler • Oct. 12, 2020 -
Regeneron follows Lilly in asking for emergency approval of COVID-19 antibody drug
The two antibody treatments are now being reviewed by the FDA, although limited supply could mean their impact won't be felt for months, even if they are cleared for use.
By Ben Fidler • Oct. 8, 2020 -
FDA moves to pull preterm birth drug from market
AMAG Pharma, the maker of Makena, said the company disagreed with the FDA's proposal and plans to evaluate its options following a failed study.
By Kristin Jensen • Oct. 7, 2020 -
FDA releases coronavirus vaccine guidelines that White House resisted
The review criteria outlined in the document, which the White House had held up for weeks, make the early approval of a coronavirus shot before the Nov. 3 election less likely.
By Ned Pagliarulo , Jonathan Gardner • Oct. 6, 2020 -
FDA told coronavirus vaccine makers of stricter standards for early approval
Blocked by the White House from issuing new vaccine guidelines, the FDA made its criteria for an emergency authorization clear anyway, publishing requirements it had previously communicated to drugmakers.
By Ned Pagliarulo • Updated Oct. 6, 2020 -
Europe begins its 2nd coronavirus vaccine review as tension builds in US
The decision by European regulators to start speedy, "rolling" reviews of two vaccine candidates comes as the FDA and White House have reportedly been unable to agree on early approval standards.
By Ben Fidler • Oct. 6, 2020 -
Solid gets all-clear from FDA to restart gene therapy trial
Worrisome immune responses had led the FDA to halt Solid's study. Now, the agency will permit Solid to continue after the biotech made adjustments to its manufacturing and patient enrollment procedures.
By Jonathan Gardner • Oct. 1, 2020 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

5 FDA approval decisions to watch in the 4th quarter
The world's attention will be on the FDA as it considers initial data from coronavirus vaccine developers. But several other important drugs, including a CAR-T therapy and an Ebola antibody, are on the agency's agenda, too.
By Ned Pagliarulo , Ben Fidler • Sept. 29, 2020