FDA: Page 9
-
Warren, Democratic lawmakers introduce bill to resurrect Chevron doctrine
The Stop Corporate Capture Act would codify the Chevron doctrine, which required federal courts to give deference to agencies’ reasonable interpretation of ambiguous statutes.
By Ginger Christ • July 25, 2024 -
FDA medical device chief Shuren to step down after 15 years
Michelle Tarver, deputy director for transformation, will serve as acting director of the FDA’s medical devices office.
By Susan Kelly , Elise Reuter • July 24, 2024 -
FDA’s lab-developed test rule could be first test of agency’s power post-Chevron
The Supreme Court’s decision to overturn the Chevron doctrine would make it easier to challenge agency regulations, such as the LDT final rule.
By Susan Kelly , Elise Reuter • July 11, 2024 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

5 FDA decisions to watch in the third quarter
Multibillion-dollar buyouts from Bristol Myers Squibb and Gilead could yield new drugs for brain and liver diseases, while a new cell therapy may reach market.
By BioPharma Dive staff • July 3, 2024 -
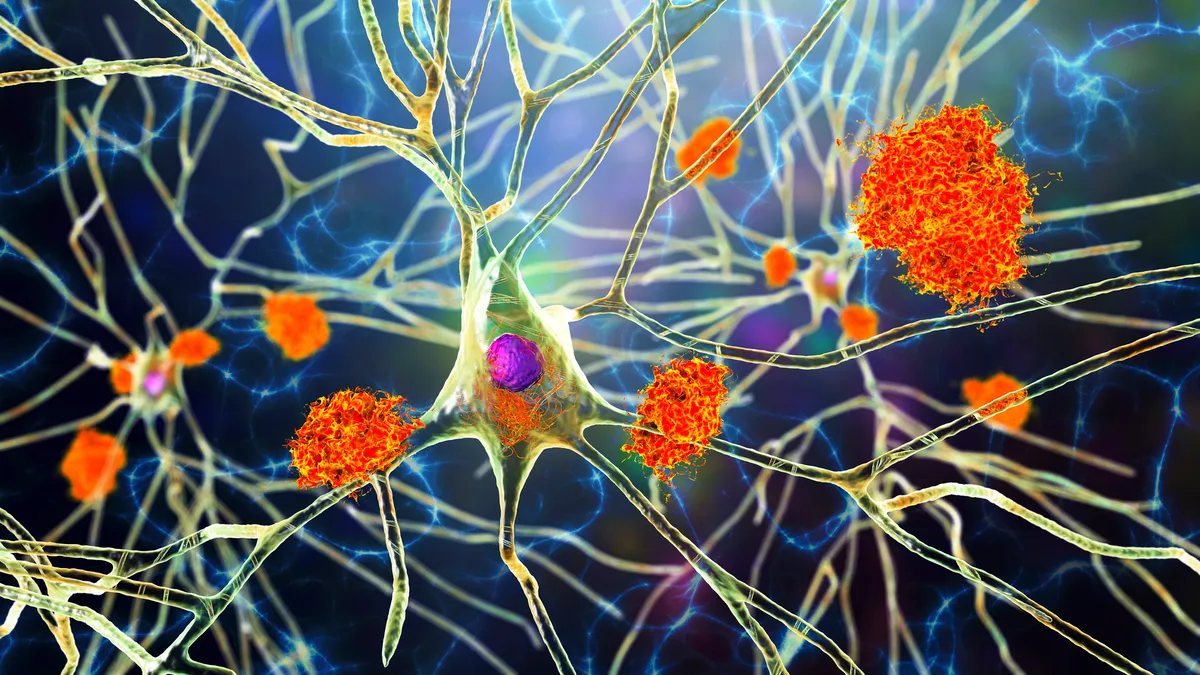
New Alzheimer's drugs
Lilly drug for Alzheimer’s approved by FDA
The drug, which Lilly will sell as Kinsunla, carries a warning for the risk of a certain kind of imaging abnormality that can be serious in rare cases.
By Jonathan Gardner • Updated July 2, 2024 -
Rocket gene therapy rejected by FDA over manufacturing
The complete response letter follows other recent manufacturing-related rejections, including one for a cell therapy from Abeona Therapeutics.
By Ned Pagliarulo • June 28, 2024 -
Supreme Court overturns Chevron doctrine, limiting reach of federal agencies
Federal courts will no longer have to defer to agency regulations for interpretation of ambiguous statutes, a ruling that could have significant impact on the FDA and CMS.
By Ryan Golden , Ginger Christ • June 28, 2024 -

 Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Duchenne approval exposes FDA rift over Sarepta gene therapy
Peter Marks’ decision to override the objections of agency staff and broaden use of Elevidys could have a “lasting impact” on gene therapy as well as the FDA, one analyst wrote at the time.
By Ben Fidler • June 21, 2024 -
Sarepta Duchenne gene therapy wins broader use from FDA
The approval makes Elevidys available to most Duchenne patients at least 4 years of age, despite mixed trial results that have led to skepticism about its effectiveness.
By Ben Fidler • Updated June 21, 2024 -
Merck wins FDA OK for vaccine rival to Pfizer’s pneumococcal shot
The new vaccine, which Merck will sell as Capvaxive, will compete with Pfizer’s blockbuster Prevnar franchise.
By Delilah Alvarado • June 18, 2024 -
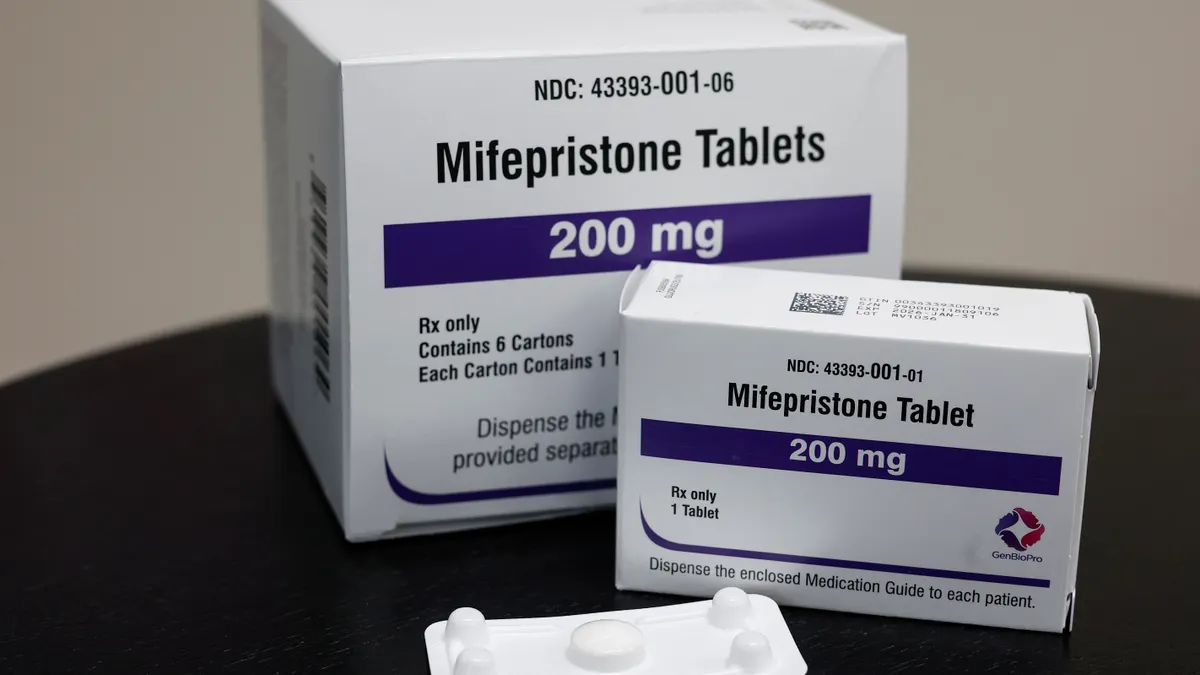
Abortion pill ruling offers measure of relief for FDA, biotech
A biotech executive called the Supreme Court’s decision a “very important win” for the industry, although new challenges to the FDA’s regulation of the drug could still be forthcoming.
By Delilah Alvarado • June 17, 2024 -
Supreme Court preserves access to abortion pill in unanimous ruling
The high court said the plaintiffs’ “desire to make a drug less available to others” did not give them standing to challenge the FDA's expansion of mifepristone’s approval.
By Delilah Alvarado • June 13, 2024 -
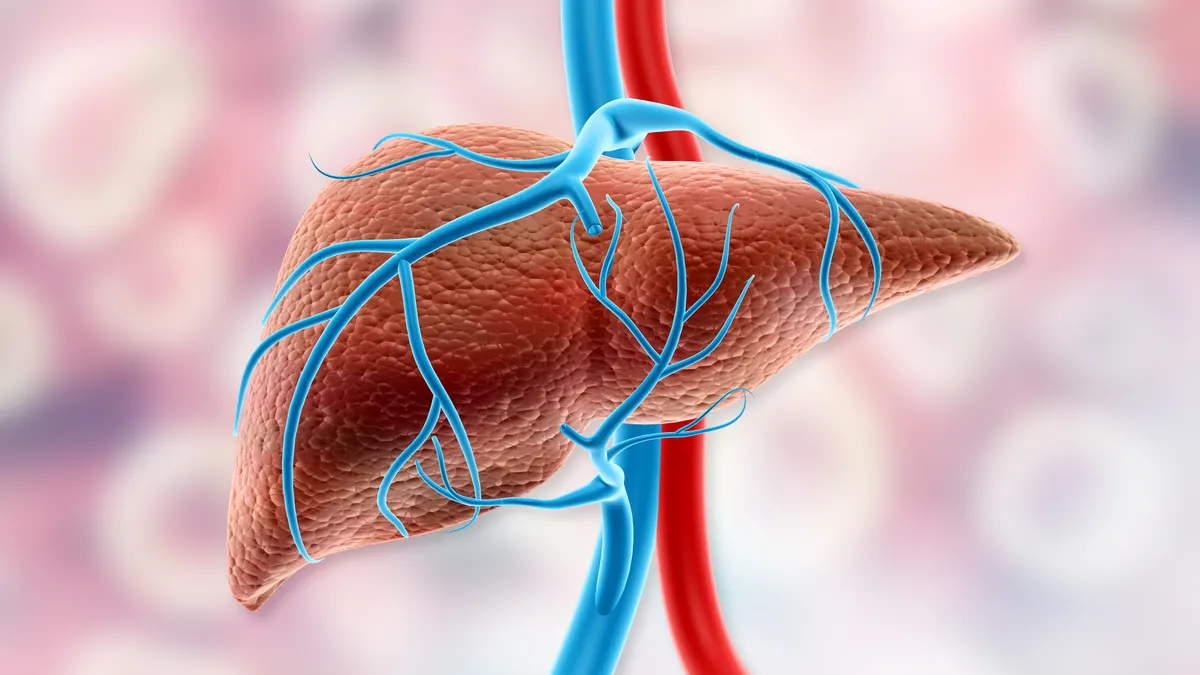
Ipsen drug for rare liver disease approved by FDA
The medicine will join Intercept’s Ocaliva as a treatment option for primary biliary cholangitis. Another drug, from the now Gilead-owned CymaBay, could be cleared for the condition by August.
By Ned Pagliarulo • June 11, 2024 -
New Alzheimer's drugs
Lilly Alzheimer’s drug gets unanimous backing of FDA panel
The expert committee's twin 11-0 votes tees up donanemab for U.S. approval later this year and a market showdown with Leqembi.
By Jacob Bell , Jonathan Gardner • Updated June 11, 2024 -
After ‘sobering’ FDA panel, psychedelics supporters wonder what’s next
A vote against MDMA-assisted therapy set back a high-profile treatment. Proponents argue the panel offered few solutions to longstanding problems, making the path forward unclear.
By Jacob Bell • June 7, 2024 -
In review of Lilly Alzheimer’s drug, FDA staff focus on safety, patient selection
An advisory panel is meeting today to discuss whether donanemab is effective across different groups of Alzheimer's patients, and to give input on an unusual dosing strategy used by Lilly.
By Jonathan Gardner • June 6, 2024 -
FDA advisers back updating COVID shots to target JN.1 virus family
The unanimous recommendation from the panel should help Novavax, which would have had difficulty adapting its shot in time if the committee picked a more specific subvariant.
By Delilah Alvarado • June 6, 2024 -
FDA panel votes against MDMA therapy for PTSD, citing muddy data and safety concerns
“I just feel very convinced that I had to vote no,” said one member of the committee, which was unconvinced by Lykos Therapeutics’ data on MDMA-assisted treatment for post-traumatic stress disorder.
By Jacob Bell • Updated June 4, 2024 -
FDA staff outline shortcomings of Lykos data for MDMA therapy
A panel of expert advisers met Tuesday to discuss several sticking points in the clinical trial results backing Lykos’ treatment for PTSD.
By Jacob Bell • May 31, 2024 -
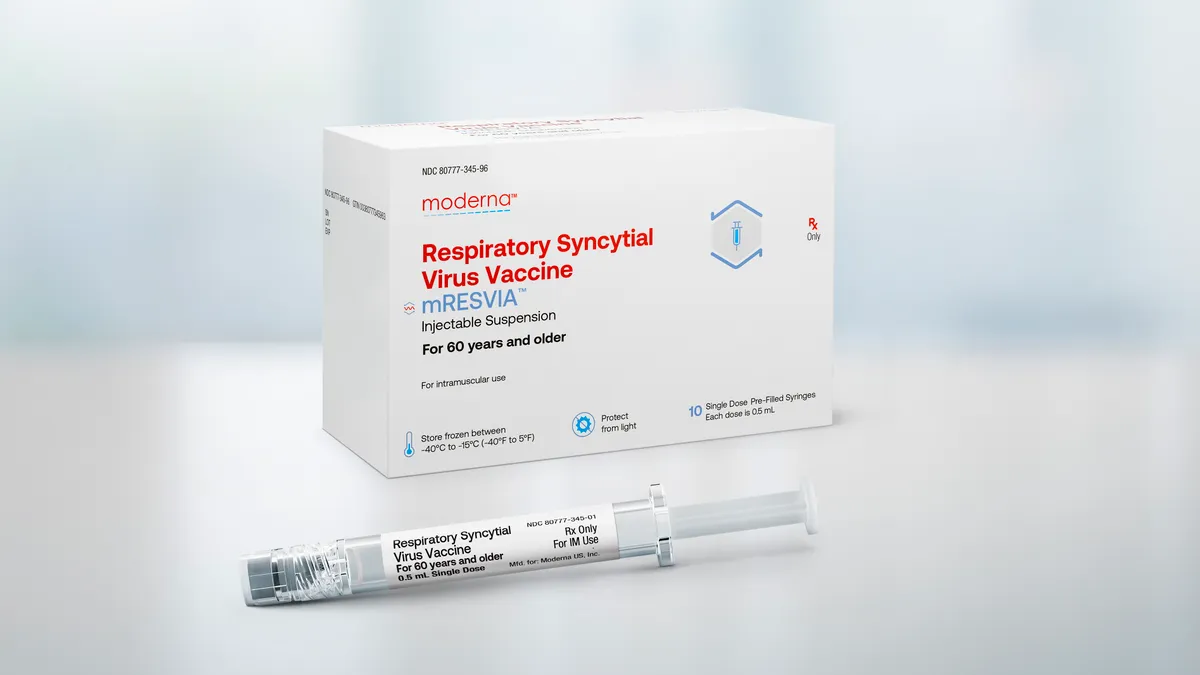
Moderna wins FDA approval for RSV vaccine
The OK for mResvia adds another option for preventing RSV-related disease in older adults, one year after the agency cleared shots from GSK and Pfizer.
By Delilah Alvarado • May 31, 2024 -

 Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
With Duchenne decision ahead, FDA’s Marks pushes for speedy gene therapy approvals
But the head of the FDA’s CBER office didn’t tip where the agency stands on potentially broadening use of Sarepta’s Duchenne gene therapy Elevidys.
By Ben Fidler • May 24, 2024 -
FDA approves Amgen drug for tough-to-treat form of lung cancer
Imdelltra, a bispecific antibody targeting a protein called DLL3, is cleared for use following chemotherapy in treating extensive-stage small cell lung cancer.
By Jonathan Gardner • May 17, 2024 -
FDA delays decision on Moderna RSV vaccine
The regulator cited “administrative constraints,” rather than any issue with Moderna’s trial data, for missing a May 12 deadline, the company said.
By Delilah Alvarado • May 10, 2024 -
Pfizer hemophilia gene therapy arrives in US to uncertain future
The Food and Drug Administration approval of Beqvez comes as other gene therapies for the bleeding condition struggle to gain traction.
By Ben Fidler • April 26, 2024 -
FDA rejects Abeona cell therapy, asks for more manufacturing data
The complete response letter for Abeona’s treatment is one of several manufacturing setbacks for cell and gene therapy developers in recent years.
By Ben Fidler • April 23, 2024