Pharma: Page 18
-
Novo sinks on obesity drug results; Sanofi reveals anticipated MS data
A pill Novo acquired produced modest weight loss but raised safety questions. Elsewhere, Sanofi detailed the silver lining it found in a recent study and a heart drug developer’s shares surged.
By BioPharma Dive staff • Sept. 20, 2024 -
Novartis’ Kisqali gets expanded FDA OK; Mene Pangalos joins Omega Funds
The FDA’s clearance could double Kisqali’s breast cancer market. Elsewhere, Novo is exploring new ways to deliver genetic therapies and Bain is funding a growing CDMO.
By BioPharma Dive staff • Sept. 18, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr. Trendline
TrendlineAlzheimer's disease
Many companies hope to follow in the footsteps of Biogen, Eisai and Eli Lilly by developing new therapies for the debilitating disease, which affects tens of millions of people and costs healthcare systems hundreds of billions of dollars each year.
By BioPharma Dive staff -
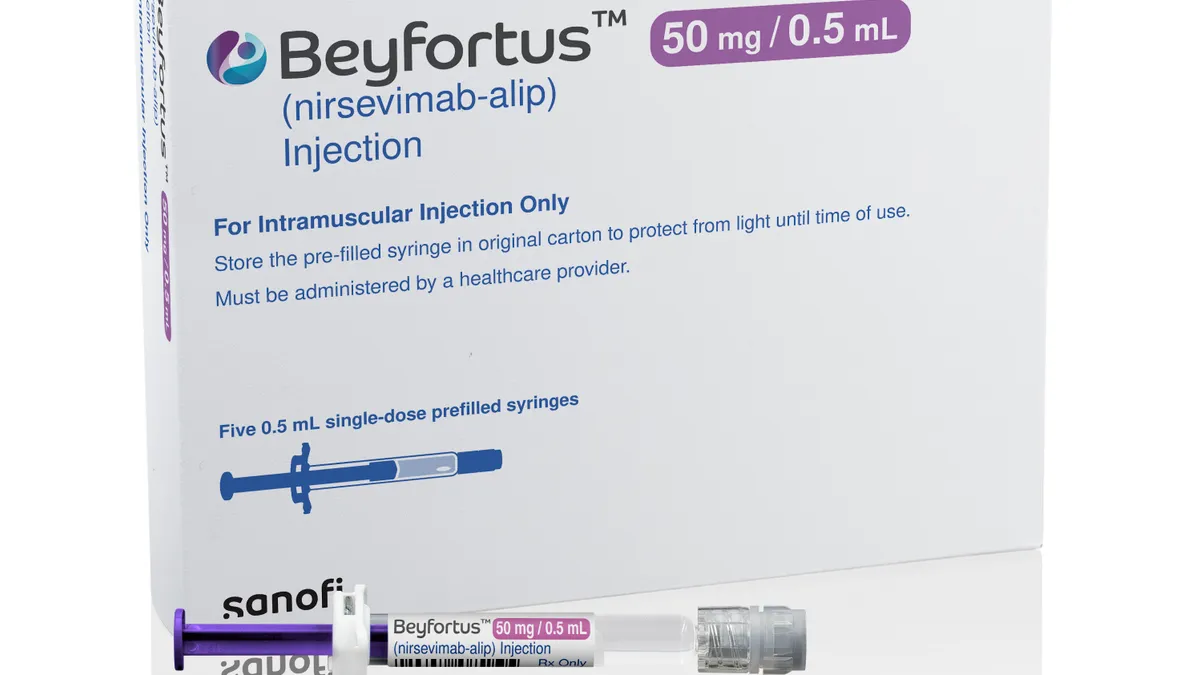
Sanofi readies to meet US demand for RSV antibody
With a new production line, Sanofi aims to keep it and partner AstraZeneca’s drug Beyfortus in full supply this RSV season.
By Delilah Alvarado • Sept. 16, 2024 -
Sponsored by SAS
Generative AI can enhance pharmaceutical innovation – but are you ready for it?
There’s no doubt that AI and GenAI can speed up pharmaceutical innovation. But before organizations can jump into the future, it’s crucial to understand the benefits – and the risks – of advanced technology.
By Susan Lenderts, Global Head of Life Sciences, SAS • Sept. 16, 2024 -
Moderna, trailing rivals, checks its RSV shot expectations
During an investor presentation Thursday, executives admitted to being overly optimistic their vaccine could wrest significant market share away from GSK's and Pfizer's products this year.
By Delilah Alvarado • Sept. 13, 2024 -
Subcutaneous Tecentriq gets FDA OK; Gilead shot succeeds in second HIV trial
Roche has now beat Merck and Bristol Myers to market with its under-the-skin immunotherapy. Elsewhere, Sanofi bought into radiopharmaceuticals and Mene Pangalos joined Biogen's board.
By BioPharma Dive staff • Sept. 13, 2024 -
Dupixent succeeds in chronic hives study, giving Sanofi, Regeneron a chance to rebound
Following an FDA rejection last year, the partners plan to resubmit their application for approval of Dupixent in chronic spontaneous urticaria.
By Kristin Jensen • Sept. 11, 2024 -
GSK discontinues herpes vaccine; Roivant launches new ‘vant’ around hypertension drug
The British pharma said its shot didn’t meet the efficacy goals of a Phase 2 study. Elsewhere, Zealand reported more obesity drug data and Lilly appointed a company veteran as its new CFO.
By BioPharma Dive staff • Sept. 11, 2024 -
Sponsored by Quest Diagnostics
Accelerating Alzheimer’s therapy innovation: A new horizon in blood-based biomarkers
The escalating global challenge of Alzheimer's Disease (AD) demands new approaches to detection, diagnosis and treatment.
By Dr. Michael K. Racke, Medical Director, Neurology, Quest Diagnostics • Sept. 9, 2024 -
Obesity drug startup raises $67M; Vor’s ‘shielded transplant’ shows promise
OrsoBio is advancing several weight loss medicines. Elsewhere, Boehringer Ingelheim pushed forward with a geographic atrophy drug and Vaxcyte raised $1.3 billion.
By BioPharma Dive staff • Sept. 6, 2024 -
Lilly builds case for weekly insulin shot
New data show Lilly’s longer-lasting insulin matched daily shots in controlling blood sugar, adding to positive findings the company disclosed in May.
By Jonathan Gardner • Sept. 5, 2024 -
High-dose Spinraza meets study goal; Top Dyne executives exit
A new dose regimen of Biogen's spinal muscular atrophy drug appeared promising, while Denali Therapeutics and Regenxbio charted plans for drug approval applications.
By BioPharma Dive staff • Sept. 4, 2024 -
Novartis builds out radiopharma production with expansion, new factory
A planned facility in California will boost Novartis’ West Coast supply chain as new uses for the company’s radioligand therapies grow.
By Kristin Jensen • Sept. 4, 2024 -
Deep Dive
A decade of cancer immunotherapy: Keytruda, Opdivo and the drugs that changed oncology
Over the past 10 years, PD1-blocking medicines have transformed cancer care. But the steady expansion of their use has slowed and, despite much trying, pharmaceutical companies have largely failed to top the drugs’ successes.
By Jonathan Gardner • Sept. 4, 2024 -
Vaxcyte’s “best-case” data for pneumococcal vaccine boost shares
The company's value jumped by several billion dollars as trial results showed its experimental shot could match and, in some cases, even outperform Pfizer's market-leading Prevnar 20.
By Delilah Alvarado • Sept. 3, 2024 -
Sanofi finds a silver lining in mixed MS drug results
Tolebrutinib, which Sanofi acquired in a $3.7 billion deal, failed two studies in people with relapsing disease, but succeeded in a type of multiple sclerosis that has no approved therapies.
By Ben Fidler • Sept. 3, 2024 -
Sponsored by Storyful
Script for success: The value of digital insights in pharma
Pharma companies can thrive in 2024 by leveraging digital intelligence to anticipate trends, analyze sentiment, and engage audiences. Discover how data-driven insights drive success.
Sept. 3, 2024 -
Novo builds heart failure case for semaglutide; Ventyx CFO departs
A new analysis showed semaglutide reduced the risk of worsening heart failure. Elsewhere, the FDA OK’d a vaccine for mpox and a biotech planned layoffs.
By BioPharma Dive staff • Aug. 30, 2024 -
Keytruda fails lung and skin cancer trials, limiting further expansion
The trial setbacks for Merck’s best-selling drug, which faces patent expiration in 2028, are a blow to the company’s plans for further indication expansion.
By Jonathan Gardner • Aug. 29, 2024 -
J&J submits touted autoimmune disease drug for FDA approval
Nipocalimab is one of several drugs J&J has forecast for multi-billion dollar sales. The company’s initial application is for its use in myasthenia gravis.
By Kristin Jensen • Aug. 29, 2024 -
Pfizer launches DTC service for migraine, COVID drugs
The platform is similar in concept to a service launched this year by Eli Lilly, and aims to give consumers easier access to Pfizer’s migraine, COVID-19 and flu treatments.
By Delilah Alvarado • Aug. 27, 2024 -
Lilly rolls out Zepbound vials at a discount price
The launch of a single-use vial form of the popular weight loss medicine could help Lilly improve supply and compete with telehealth companies offering compounded versions.
By Ned Pagliarulo • Aug. 27, 2024 -
RA Capital-backed startup raises $100M; UCB sells China business
New biotech Navigator is working on drugs for immune diseases. Meanwhile, UCB sold its neurology and allergy business in China, and J&J's Peter Fasolo will retire this year.
By BioPharma Dive staff • Aug. 27, 2024 -
Regeneron gains European approval for bispecific lymphoma drug
Regulators in Europe granted approval based on remission rates, while the FDA sought more progress in confirmatory trials when it rejected Ordspono.
By Jonathan Gardner • Aug. 26, 2024 -
FDA to convene immunotherapy panel; Tome scales back operations
Agency advisers will discuss whether use of Keytruda and Opdivo should be limited in gastric cancer. Elsewhere, a buzzy genetic drug startup is exploring “strategic options.”
By BioPharma Dive staff • Aug. 23, 2024