Pharma: Page 28
-
Pfizer RSV vaccine gets off to fast start on market
The pharma’s shot Abrysvo made over $300 million in sales during the first few months of its launch, and will be an important product to help offset declining COVID-19 vaccine sales.
By Delilah Alvarado • Oct. 31, 2023 -
Novartis bet on kidney disease drug yields positive study data
The pharma paid more than $3 billion this summer to acquire Chinook Therapeutics and a therapy that just met its goal in a Phase 3 trial.
By Ned Pagliarulo • Oct. 30, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr. Trendline
TrendlineAlzheimer's disease
Many companies hope to follow in the footsteps of Biogen, Eisai and Eli Lilly by developing new therapies for the debilitating disease, which affects tens of millions of people and costs healthcare systems hundreds of billions of dollars each year.
By BioPharma Dive staff -
Sponsored by Flatiron
Why prospective real-world studies hold so much promise for clinical research
PrwS studies can reach beyond the limitations of today’s RWE studies and expand the role of RWD across the entire drug development cycle.
By Josh Buddle, Director of Clinical Operations, Flatiron Health • Oct. 30, 2023 -
Q&A
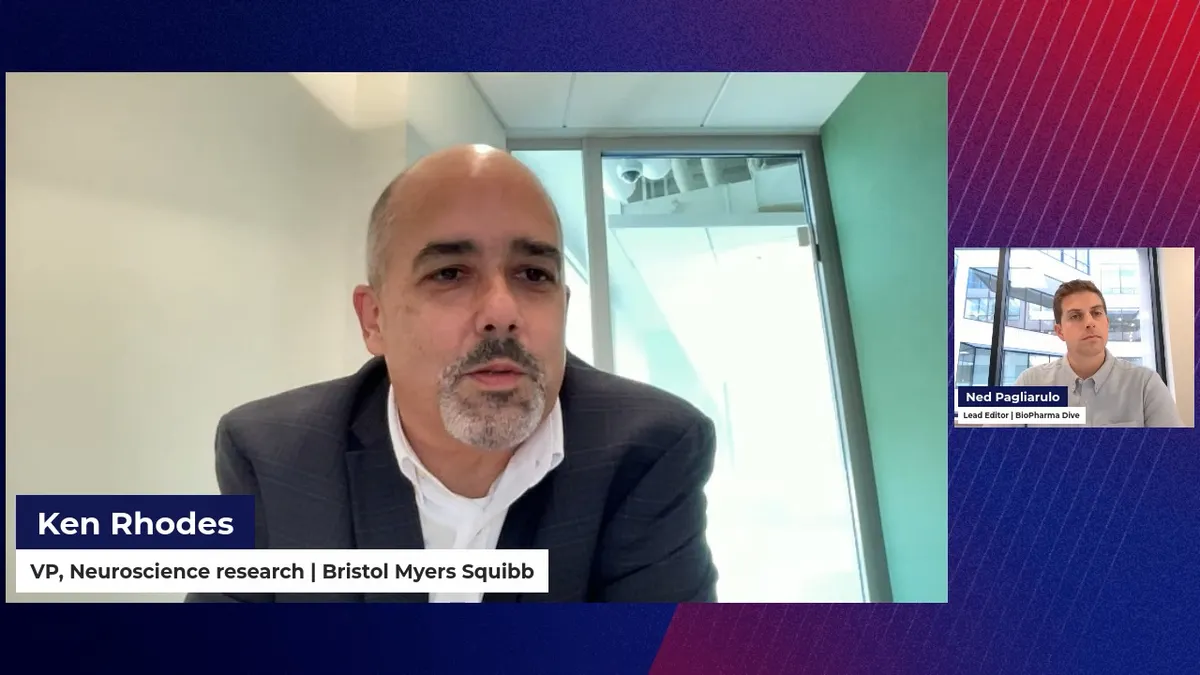
Bristol Myers’ neuroscience research head explains the big pharma’s return to brain drugs
Bristol Myers’ Ken Rhodes spoke with BioPharma Dive about biomarkers, diseases of interest and why the company is far from done with neuroscience-focused deals.
By Jacob Bell , Ned Pagliarulo • Oct. 27, 2023 -
Sanofi to divest consumer unit, joining pharma industry shift
The restructuring came as the French company forecast an earnings drop in 2024, prompting a stock selloff that erased about $20 billion in market value.
By Jonathan Gardner • Oct. 27, 2023 -
Bristol Myers says new drugs sales will grow more slowly
Company shares fell Thursday as the company adjusted its medium-term guidance for sales of new products like Reblozyl, Camzyos and Abecma.
By Ned Pagliarulo • Oct. 26, 2023 -
Amgen to lay off 350 Horizon staffers weeks after closing $28B deal
The latest round of cuts brings the company’s total announced workforce reductions this year to more than 1,000.
By Kristin Jensen • Oct. 25, 2023 -
Novartis delays FDA filing for in-demand radiopharma drug
Mixed survival data from a study of Pluvicto in earlier prostate cancer sparked the slower regulatory timeline.
By Jonathan Gardner • Oct. 24, 2023 -
J&J medtech head Ashley McEvoy to step down
McEvoy is leaving the company after 27 years to pursue other opportunities. Tim Schmid will now lead the MedTech business.
By Elise Reuter • Updated Oct. 24, 2023 -
Sponsored by Target RWE
Leveraging real-world evidence with analytics: 3 essential components to capture a 360-degree view of your patient population
Three essential components to create impactful real-world evidence with a 360-degree patient journey.
By Ewa Kleczyk, PhD, SVP, Commercial Analytics & Data Curation • Oct. 23, 2023 -
Merck allies with Daiichi Sankyo in major bet on antibody cancer drugs
The deal is worth up to $22 billion in total, making it one of the largest pharmaceutical licensing agreements by value.
By Ned Pagliarulo • Oct. 20, 2023 -
J&J again bumps forecasts on strong immune drug sales
Tuesday’s earnings were the first since J&J officially split off its consumer drug business, now the publicly traded company Kenvue.
By Ned Pagliarulo • Oct. 17, 2023 -
The top biopharma conferences in 2024
A few key meetings remain this year, like AHA and ASH, while next year’s events are already being planned. Here’s a list of conferences to keep in mind.
By Ned Pagliarulo • Oct. 16, 2023 -
Pfizer to cut costs, lay off staff on waning demand for COVID products
Sales of Pfizer’s pill Paxlovid and shot Comirnaty have been slower than it anticipated, while a shift to the commercial market for the antiviral drug has been delayed. Shares rose Monday on the reset guidance.
By Ned Pagliarulo • Updated Oct. 16, 2023 -
Pfizer wins FDA approval for its $7B colitis drug
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
By Jonathan Gardner • Oct. 13, 2023 -
Research group says FDA found no misconduct in Pfizer Lyme vaccine trial it helped run
Earlier this year Pfizer removed thousands of participants from a study of its Lyme disease shot over concerns the group, Care Access, wasn't meeting clinical practice standards.
By Delilah Alvarado • Oct. 12, 2023 -
Bayer opens California plant to make cell therapies
The pharma plans for the new Berkeley facility to produce clinical, and potentially commercial, supplies of a Parkinson’s disease cell therapy being tested by its subsidiary BlueRock.
By Delilah Alvarado • Oct. 10, 2023 -
GSK partners with Chinese pharma to expand Shingrix sales
The purchase agreement with Chongqing Zhifei Biological Products is part of GSK’s plan to double sales of the shingles vaccine by 2026.
By Delilah Alvarado • Oct. 9, 2023 -
Apellis sales numbers show steady demand for new eye drug, despite safety worries
The company says Syfovre prescriptions accelerated again in August after a rocky summer of side effect probes.
By Kristin Jensen • Oct. 5, 2023 -
Lilly names new diabetes and obesity chief as Mounjaro sales take off
Company veteran Mike Mason, who took leadership of the division four years ago, is retiring and will be replaced by immunology head Patrik Jonsson.
By Jonathan Gardner • Oct. 4, 2023 -
Moderna claims positive results in early study for combo COVID, flu shot
The company is planning to start a Phase 3 trial of the vaccine this year, and is targeting a regulatory approval in 2025.
By Delilah Alvarado • Oct. 4, 2023 -
Sandoz spins out of Novartis as standalone generic drugmaker
The spinoff, now complete, is a major step in CEO Vas Narasimhan’s plan to refocus Novartis more tightly around novel prescription drugs and new technologies.
By Kristin Jensen • Oct. 4, 2023 -
J&J, retreating from infectious disease, partners with Sanofi on E. coli vaccine
The French vaccine giant will pay J&J $175 million to share rights to the experimental inoculation, which is currently in Phase 3 testing.
By Kristin Jensen • Oct. 3, 2023 -
Lilly to enter radiopharmaceutical drug field with $1.4B Point buyout
Point Biopharma is one of the most advanced in a group of emerging biotechs working on radioligand therapies for cancer.
By Ned Pagliarulo • Oct. 3, 2023 -
Novartis’ closely watched rare disease drug scores in kidney disorder
Called iptacopan, the experimental medicine has now scored positive results in three late-stage clinical trials, the latest in an uncommon kidney disorder known as IgA nephropathy.
By Jacob Bell • Oct. 2, 2023