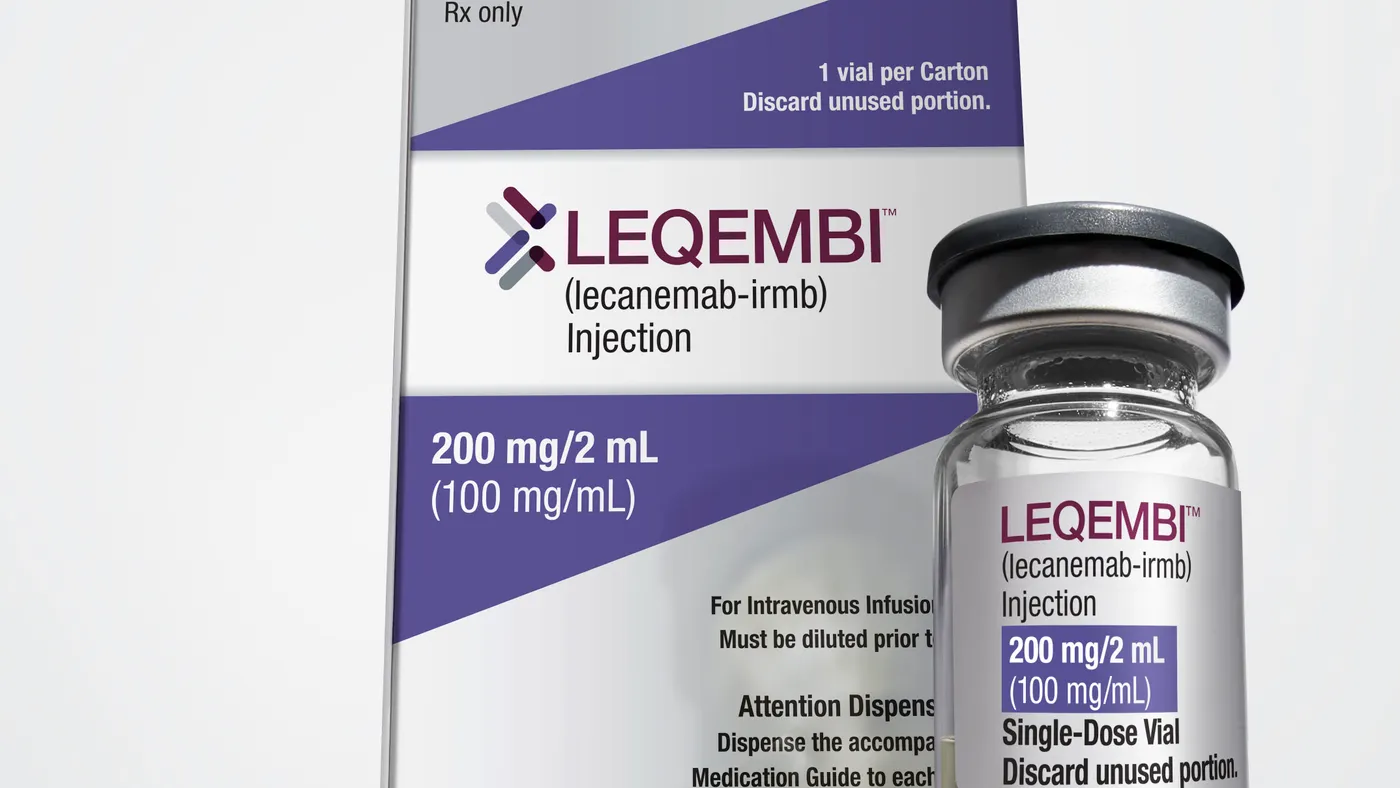
Biogen ‘convinced’ the worst is over for Leqembi
After a slow start, the launch of Biogen’s prized drug for Alzheimer’s disease is picking up, which has given company leadership more confidence that it can both drive growth and compete with a rival medicine from Eli Lilly.
“We’re pretty convinced Leqembi is on the right path now,” CEO Chris Viehbacher said on a conference call with reporters.
Biogen’s development partner Eisai, which is spearheading Leqembi’s launch, had made optimistic predictions about the drug’s early launch trajectory. Biogen executives have been hesitant to second those estimates, noting that growth will depend on whether large, integrated healthcare systems adopt Leqembi.
Yet, if orders from these systems are any indication, Leqembi is on track to becoming a big product in the long-term, Viehbacher said. After an earlier setback, Eisai and Biogen also secured a long-awaited approval in Europe, a market some analysts believe could contribute about 20% of future sales.
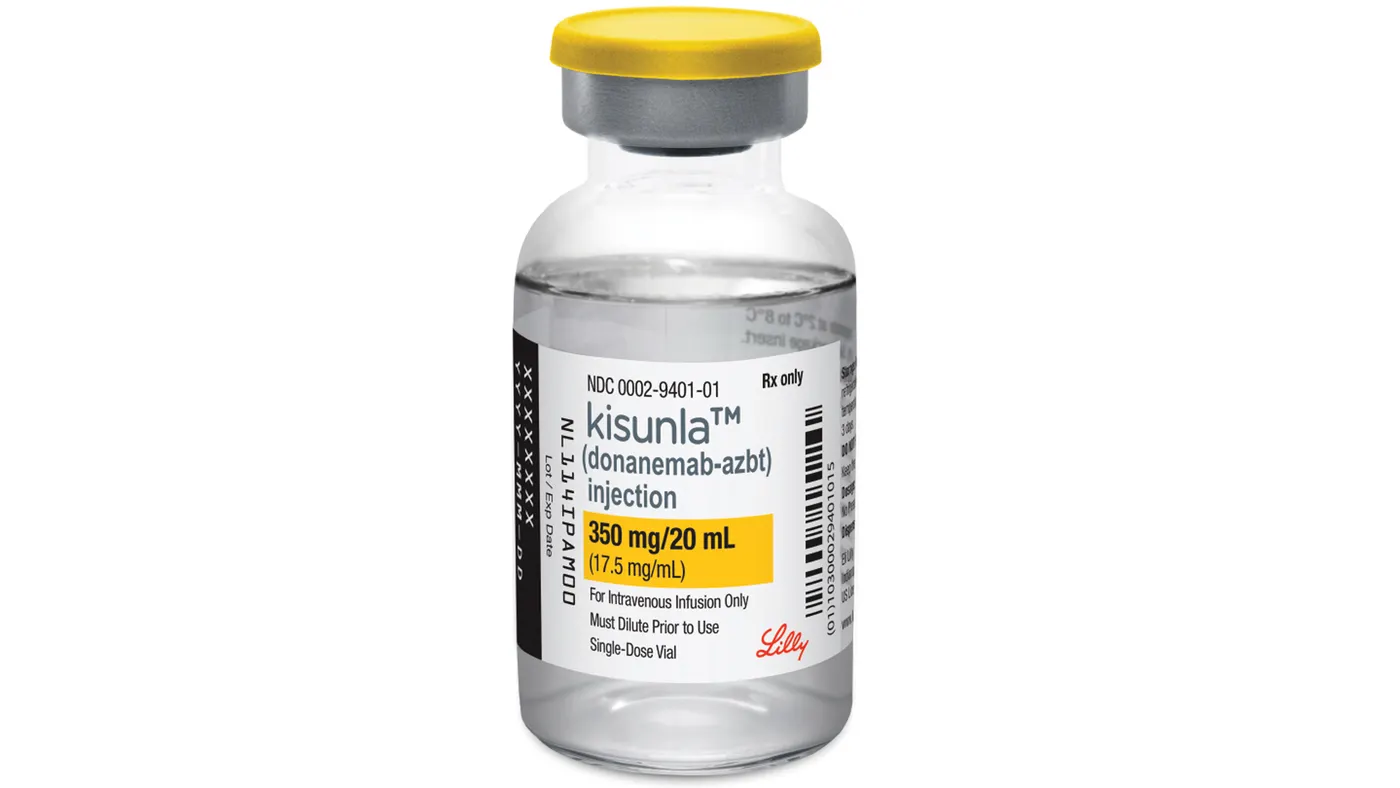
Until recently, Leqembi and Aduhelm — another drug developed by Eisai and Biogen — were the only Alzheimer’s therapies of their kind cleared in the U.S. They work by removing from the brain toxic clumps of a protein called amyloid beta. But that list lengthened with the approval of Lilly’s anti-amyloid medicine Kisunla.
Analysts have been debating which drug, Leqembi or Kisunla, will take the lead in a rapidly evolving market. Both come as intravenous infusions, and are specifically for Alzheimer’s patients in the early stages of the disease. There are key differences, however.
Data suggest Leqembi may be safer, while Kisunla is seen as more convenient since it’s administered every four weeks instead of every two. And because of the tests Lilly ran, patients can stop taking Kisunla once their amyloid levels drop below a certain threshold.
Biogen and Eisai believe a newer form of Leqembi they’ve been developing, an under-the-skin shot that’s also been approved, will help lift prescriptions and sales. Additionally, a large, late-stage clinical trial is evaluating Leqembi in people who have elevated amyloid levels but aren’t yet showing Alzheimer’s symptoms.
“Only Leqembi is really going to have the evidence base that covers that whole spectrum, from the early stage right through to maintenance,” Viehbacher said. Kisunla “is not going to be able to play into that.”
“Short term, we're going to see competition, certainly,” he added. “But I would also say that one of the biggest challenges to this launch has been building up the healthcare infrastructure. And so in a lot of ways, we actually welcome Lilly’s entry into the market, because [the] extra education that will happen with healthcare professionals will grow the market for everyone.”