The Latest
-
Deep Dive // Emerging biotech
Biotech IPOs are the industry’s lifeblood. Track how they’re performing.
New biotech stock offerings in the U.S. bottomed out in 2025, with only 11 drugmakers raising about $1.6 billion in IPO proceeds — the lowest total in at least seven years.
Updated Dec. 22, 2025 -
News roundup
Trump to test ‘most favored nation’ pricing for Medicare; Shionogi pays $2.5B for ALS drug
Two pilot programs would tie Medicare costs and rebates in the U.S. to what’s paid in comparable countries. Elsewhere, AstraZeneca reported a setback and an “off the shelf” cell therapy showed promise.
-
BioMarin to buy rare disease drugmaker Amicus for $4.8B
Analysts said the deal makes sense for BioMarin, as it offers a late-stage clinical asset as well as two marketed therapies that generated nearly $450 million over the first nine months of this year.
-
Patient deaths put Merck, Daiichi’s ADC trial on partial hold
The FDA placed the hold after researchers recorded a “higher than anticipated” incidence of deaths in a study testing “I-DXd” in people with small cell lung cancer.
-
Takeda says $4B TYK2 drug succeeds in large psoriasis studies
The results position Takeda, which acquired its therapy in one of the industry’s larger single-drug acquisitions, to challenge Bristol Myers Squibb’s Sotyktu.
-
Galapagos TYK2 drug hits goal in one trial, misses in another
The Belgian biotech is still on the hunt for partners after reporting that its drug succeeded against an inflammatory skin and muscle disorder but missed in a lupus study.
-
China competition
Drugs from China are reshaping biotech. Track the licensing deals here.
U.S. and European companies announced at least 62 drug licensing deals with Chinese companies in 2025, the largest five of which topped $4 billion in overall value.
Updated Dec. 22, 2025 -
News roundup
Novo files for CagriSema approval; Merck and Pfizer’s trial win
Novo Nordisk's application for CagriSema comes at the same time as competitor Eli Lilly's ask for its obesity drug orforglipron. Elsewhere, Merck & Co. and Pfizer succeeded in a bladder cancer study.
-
Insmed’s ‘win streak’ ends as top drug fails study in chronic nasal condition
The company’s valuation, which surged past $40 billion this year, fell by almost a fifth on a dimmer outlook for its closely followed lung disease medicine.
-
Emerging biotech
Orum, a biotech marrying ADCs with protein degraders, captures $100M in funding
The financing follows a strategic shift for Orum, which shelved its original lead program and is now pursuing a potential blood cancer treatment.
-
Peanut allergy patch succeeds in late-stage study 5 years after FDA rejection
DBV Technologies plans to use the latest Phase 3 results in a new submission to U.S. regulators in 2026.
-
Deep Dive
Biotech’s M&A outlook is uncertain. Track the deals that are happening here.
Biopharma dealmakers spent over than $112 billion on acquisitions last year, more than doubling 2024’s total.
Updated Dec. 22, 2025 -
Startup launches
Addition emerges with $100M to make gene therapies for chronic and rare diseases
Backed by a handful of prominent investors including the Gates Foundation, the startup is pursuing an approach it believes can sidestep issues seen with traditional gene therapy technology.
-
Vaccines
Moderna gets funding for H5 pandemic influenza vaccine
The fresh capital provides another shot for Moderna's candidate against bird flu, which earlier this year lost its government funding.
-
FDA clears GSK’s twice-yearly asthma drug
Exdensur is now the first asthma biologic to be approved for twice-yearly dosing, and could potentially change standard of care for those with severe disease.
Updated Dec. 17, 2025 -
Deep Dive // Emerging biotech
Biotech startups are built on venture capital. Track funding rounds here.
After a slow start, venture capital funding involving the firms tracked by BioPharma Dive surged back to life toward the end of 2025, bringing the amount raised close to 2024’s total.
Updated Dec. 11, 2025 -
Pain drugs
RA Capital backs drug for rare kind of chronic pain
The investment firm co-led a $125 million Series A round for Ambros Therapeutics, a Vivek Ramaswamy co-founded company with a medicine nearing pivotal testing.
-
Biotech zombies
‘Zombie’ biotech buyer Xoma to acquire Generation Bio
The gene therapy developer has lost most of its value since going public in 2020 and saw a long road ahead for a delivery technology that had shown promise in preclinical tests.
-
Nektar sees silver lining as autoimmune drug ‘narrowly’ misses in alopecia study
Despite being unable to declare statistical success, the company claimed its therapy showed enough promise to support moving into late-stage testing.
-
News roundup
FDA ‘proactively’ hands J&J a voucher; Pfizer issues 2026 forecasts
The FDA contacted J&J right after ASH to discuss using a special fast pass on a multiple myeloma regimen. Elsewhere, a new cell therapy player emerged with $60 million in Series A funding.
-
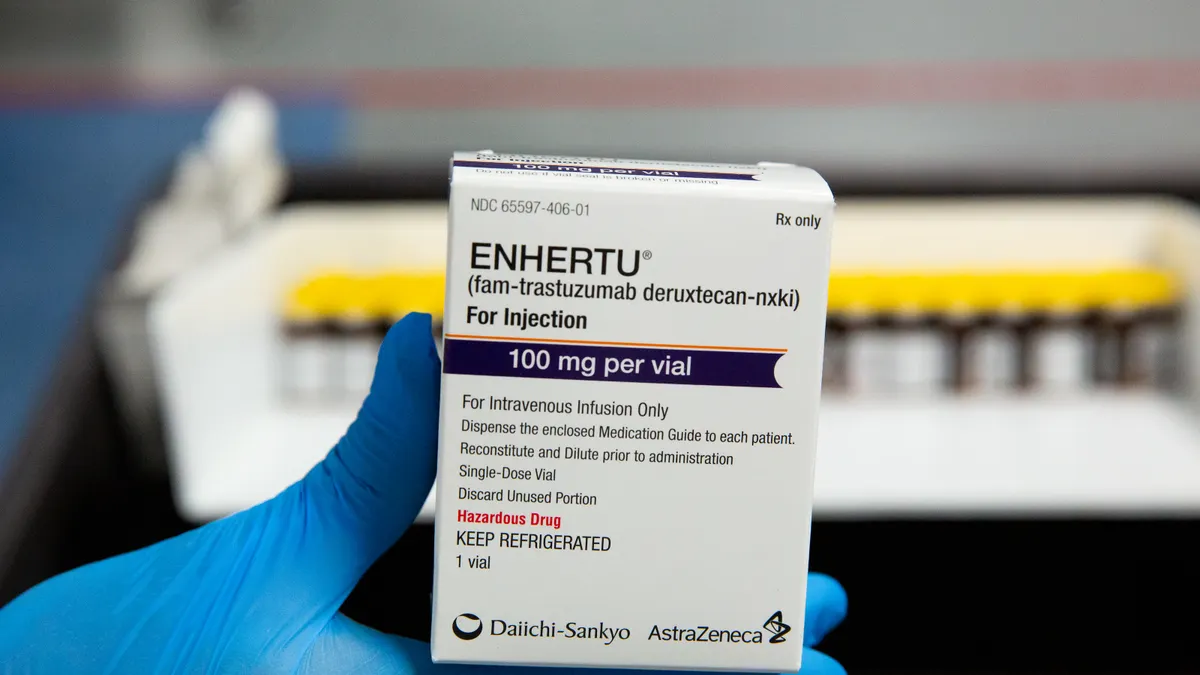
Enhertu combo cleared for use in frontline breast cancer
The approval could help Enhertu, which is already a multibillion-dollar seller, supplant a regimen that’s been the standard of care for HER2-positive breast tumors for more than a decade.
-
Sanofi MS drug hits two setbacks
Tolebrutinib, which Sanofi acquired in a $3.7 billion buyout, failed a Phase 3 study in “primary progressive” multiple sclerosis and is facing a delayed U.S. approval decision in another form of the disease.
-
News roundup
Lundbeck sparks bidding war with Alkermes; Pfizer closes Metsera deal
Pfizer quickly consummated the $10 billion buyout after one-upping Novo Nordisk. Elsewhere, Lundbeck submitted a rival offer for Avadel Pharmaceuticals and Zealand Pharma dropped an obesity drug.
-
Immune reset
Kyverna to seek first clearance of a CAR-T therapy for autoimmune disease
The company intends to file a U.S. application in the first half of 2026 following positive study results in a condition called stiff person syndrome that has no approved therapies.
-
Argenx falters in effort to expand immune drug’s use
The company will stop two trials testing its blockbuster therapy Vyvgart in thyroid eye disease after treatment was judged to be ineffective at an interim checkpoint.