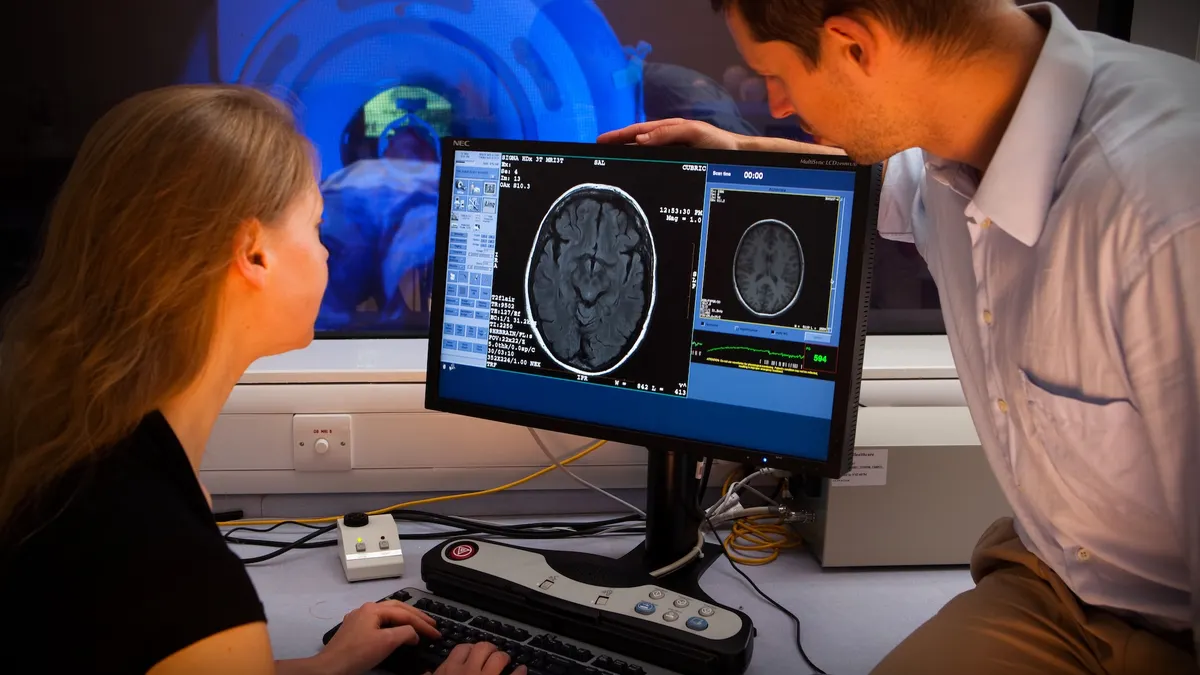
Shares of Swiss drugmaker AC Immune rose by double-digits Thursday after the company reported positive data from a clinical trial testing its experimental vaccine for Parkinson’s disease.
The vaccine is designed to spur the immune system to produce certain antibodies that can both break up and stop the spread of toxic clumps of “alpha-synuclein,” a protein closely linked to Parkinson’s. An ongoing, mid-stage trial has enrolled around 150 people at early stages of the disease, and is comparing three different doses of the immunotherapy against a placebo over a main study period of a year and a half.
The first part of the experiment includes 34 participants who’ve been treated for somewhere between 12 and 18 months. According to AC Immune, interim results show that not only is its therapy triggering the desired immune response, but biological markers as well as clinical measures of motor function suggest it may also be stabilizing patients’ disease. Movement issues like muscle shaking and difficulty balancing are hallmark symptoms of Parkinson’s.
The company noted, for example, how levels of a protein tied to nerve cell damage had increased in the placebo group but remained steady in the vaccine arms. For brain drug developers, demonstrating an effect on this “neurofilament light chain” protein has become more important over the past few years, ever since Biogen used that measurement to secure approval for an ALS medicine.
AC Immune claims these results show, for the first time, that the progression of Parkinson’s could be slowed by using an active immunotherapy to target disease-causing alpha-synuclein.
"The remarkable consistency of the trends observed across multiple disease-related biomarkers and on clinical assessments in the treatment arm are very promising,” said Werner Poewe, an emeritus professor of neurology at Innsbruck Medical University in Austria, in a statement from AC Immune.
“Overall, these findings are highly encouraging and fully support further development of the program,” Poewe added.
AC Immune said data collected at the trial’s 50-week and 76-week marks affirm that the immunotherapy is generally safe, well-tolerated, and that its benefits appear to outweigh the possible risks. Researchers have yet to report any “clinically relevant or serious adverse events” related to the shot. Transient injection site reactions were the most common adverse event, occurring in a little more than half of participants.
Fatigue and headaches were also documented in 12% and 15% of patients, respectively.
This immunotherapy has the potential to be a “tremendous step forward for millions of patients,” said AC Immune’s CEO Andrea Pfeifer. The safety and effectiveness seen so far create a “strong basis for accelerating development.”
Encouraged by these latest results, AC Immune intends to ask drug regulators for feedback on its development plans, to “potentially accelerate” the time to an approval filing. Final data from the first part of the Phase 2 trial are expected in the middle of next year.
Company shares, which trade on the Nasdaq, were up almost 14% by mid-day Thursday, to $3.17 apiece.
With its stock price suppressed for much of the past three years, AC Immune recently conducted a strategic review that led the company to narrow its research lens and lay off around 30% of its workforce. AC Immune determined that, moving forward, it’ll prioritize three clinical-stage immunotherapy programs, plus some of its “most promising” small molecule drugs.
The company has said these reductions should save enough money to keep it operational until at least the third quarter of 2027.
The Parkinson’s vaccine is one of AC Immune’s few wholly-owned clinical programs.