Biotech: Page 23
-
Novocure device succeeds in pancreatic cancer study; FDA sets Cytokinetics decision date
Using tumor-treating fields alongside chemotherapy improved patient survival, Novocure said. Elsewhere, Cytokinetics got a key PDUFA date and Fate Therapeutics swapped CEOs.
By BioPharma Dive staff • Dec. 2, 2024 -
Pharma hopes Trump will bring change to the FTC. They may be disappointed.
Some in the drug industry expect more lenient merger enforcement under a second Trump administration. One former regulator argues that might not be the case.
By Kelly Bilodeau • Nov. 27, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
Q&A // Brain drug revival
How Intra-Cellular surprised Wall Street by breaking character
The biotechnology company, which sees itself as "pretty conservative," recently put out an ambitious long-term revenue forecast for its schizophrenia and depression medicine Caplyta.
By Jacob Bell • Nov. 27, 2024 -
Kronos, Idorsia plan layoffs; PTC shelves ALS drug
Kronos is cutting 83% of its workforce, while Idorsia is considering eliminating as many as 270 jobs. Elsewhere, PTC disclosed negative Phase 2 results for its ALS drug candidate utreloxastat.
By BioPharma Dive staff • Nov. 27, 2024 -
Biohaven muscle drug misses goal of SMA study, but advances in obesity
The setback likely removes one competitor to an emerging spinal muscular atrophy medicine from Scholar Rock, but sets the stage for a pair of readouts with implications for weight loss research.
By Ben Fidler • Nov. 25, 2024 -
BridgeBio heart drug approved by FDA, setting up battle with Pfizer
BridgeBio shares climbed by nearly 25% Monday on news its drug for a cardiac form of transthyretin amyloidosis was approved with a broad label that looks competitive to Pfizer's tafamidis.
By Ben Fidler • Nov. 22, 2024 -
Halozyme drops Evotec buyout bid; Patient dies in Neurogene trial
Evotec appears to have rebuffed Halozyme’s $2.1 billion acquisition attempt. Elsewhere, Argenx has another blockbuster target in sight and an FDA veteran plans retirement.
By BioPharma Dive staff • Nov. 22, 2024 -
Lexicon to disband sales team, lay off 60% of staff
The restructuring follows the company's receipt of a letter from the FDA citing "deficiencies" in its approval application for diabetes drug Zynquista.
By Ned Pagliarulo • Nov. 22, 2024 -
Deep Dive // Emerging biotech
Biotech startups are built on venture capital. Track funding rounds here.
Corxel’s $287 million round ranks as the second-largest this month involving firms tracked by BioPharma Dive, trailing only Parabilis Medicines’ $305 million haul.
By Gwendolyn Wu , Ben Fidler , Ned Pagliarulo , Julia Himmel • Updated Jan. 23, 2026 -
Amgen picks prolific biotech founder Chang as new top scientist
Howard Chang will take over as chief scientific officer and senior VP of research as the biotech searches for more ways to overcome the looming loss of exclusivity for some of its top-selling medicines.
By Kristin Jensen • Nov. 21, 2024 -
Versant startup sets out to make a new type of obesity drug
The startup, Pep2Tango, is combining four methods of accelerating weight loss into one medicine in the hopes of developing a treatment that can improve upon drugs like Wegovy and Zepbound.
By Gwendolyn Wu • Nov. 21, 2024 -
Sage’s string of research failures continues
Negative results from a Huntington’s trial add to a calamitous year for Sage, which last month decided to overhaul its research, reconfigure its executive team and lay off a third of its staff.
By Jacob Bell • Nov. 20, 2024 -
Chasing new ‘checkpoints,’ startup Valora emerges from a Nobel winner’s lab
Built around research by Stanford scientist Carolyn Bertozzi and MIT researcher Jessica Stark, Valora Therapeutics is designing drugs to target glyco-immune checkpoints.
By Gwendolyn Wu • Nov. 20, 2024 -
Flagship, Pfizer alliance yields two more startup deals
Pfizer will work with Ampersand Biomedicines and Montai Therapeutics to find drugs for obesity and lung cancer, respectively, adding to collaborations it previously formed with other Flagship startups.
By Gwendolyn Wu • Updated Nov. 20, 2024 -
Astellas application rejected by FDA; Cytokinetics strikes licensing deal with Bayer
The agency turned back Astellas’ attempt to update its drug Izervay’s labeling. Elsewhere, former NCI director Ned Sharpless founded a new startup and Novartis licensed another radiopharma drug.
By BioPharma Dive staff • Nov. 19, 2024 -
Incyte sinks on setback for drugs acquired in $750M buyout
The company paused testing of one candidate acquired in its April deal for Escient and scrapped another in a blow to its diversification plans.
By Ben Fidler • Nov. 19, 2024 -
Gene editing
CRISPR therapy from Intellia may ameliorate rare heart disorder, data suggest
Phase 1 data indicate Intellia’s medicine could be a powerful treatment for a cardiac form of ATTR amyloidosis. But rival drugs are further ahead.
By Ned Pagliarulo • Nov. 18, 2024 -
Q&A // Brain drug revival
How a Biogen drug set the stage for a new biotech targeting ALS
Trace Neuroscience, which launched this week with $101 million, benefited from last year’s approval of Qalsody. Its CEO spoke with BioPharma Dive about how the company came together.
By Jacob Bell • Nov. 15, 2024 -
‘Find any advantage’: 3 biotech leaders on driving R&D success
“Many companies just iterate indefinitely without ever making progress toward an actual drug,” said one biotech VC on a panel hosted by BioPharma Dive.
By Jonathan Gardner • Nov. 15, 2024 -
Emerging biotech
Surviving in biotech’s new normal: 5 tips from industry VCs and CEOs
At an event hosted by BioPharma Dive, drugmaker executives and investors discussed the importance of focus, smart spending and maintaining lines of sight to the clinic.
By Ned Pagliarulo , Delilah Alvarado • Nov. 15, 2024 -
Halozyme bids for Evotec; BeiGene gets a new name
The San Diego biotech made an unsolicited bid to buy Evotec for 2 billion euros. Elsewhere, Bluebird recorded its first Lyfgenia revenue and Leerink built up its M&A team.
By BioPharma Dive staff • Nov. 15, 2024 -
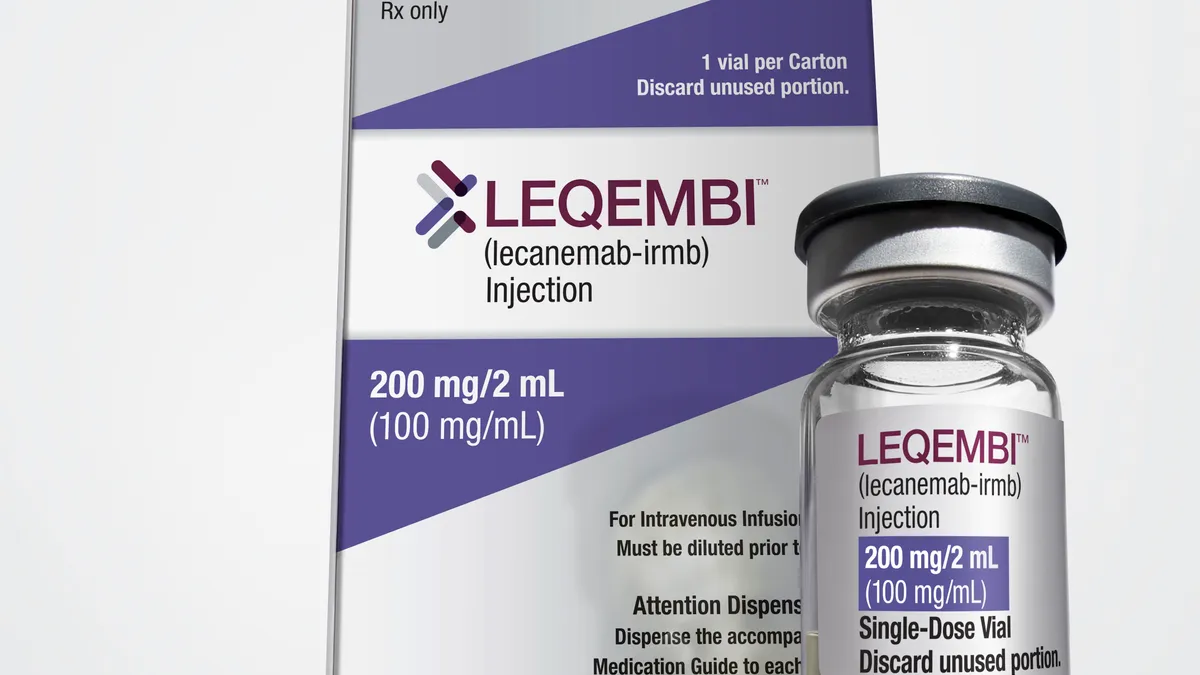
New Alzheimer's drugs
Eisai wins over European regulators on Alzheimer’s drug Leqembi
The EMA sided against the drug this summer. But an appeal from Eisai appears to have worked, teeing Leqembi up for authorization in a major market.
By Jacob Bell • Nov. 14, 2024 -
Merck, facing threat to Keytruda, buys into new kind of cancer immunotherapy
The company is paying China-based biotech LaNova Medicines $588 million for the same type of bispecific antibody drug that recently bested Keytruda in a clinical trial.
By Ben Fidler • Nov. 14, 2024 -
23andMe to lay off more than 200 employees amid business struggles
The genetic testing firm will also close its drug development arm. CEO Anne Wojcicki said the cuts are necessary to “focus on the long-term success of our core consumer business.”
By Ricky Zipp • Nov. 13, 2024 -
Amgen shares sink on obesity drug concerns; AstraZeneca to spend $3.5B on manufacturing
An analyst note raising questions about Amgen’s MariTide triggered a stock sell-off Tuesday. Elsewhere, Roche and Novartis struck drug discovery deals.
By BioPharma Dive staff • Nov. 13, 2024