Biotech: Page 52
-
Sponsored by Vetter Pharma
How to successfully bring your drug product to launch
Vetter brings a proven launch management process that combines extensive experience with in-depth product knowledge.
By Dr. Anna Schamberger, Director Customer Project Management & Sabrina Schumacher, Team Leader Customer Service • May 15, 2023 -
Amylyx’s ALS drug again surpasses Wall Street expectations
Revenue from the biotech’s ALS therapy Relyvrio totaled $71.4 million in the first quarter, well above analyst estimates and helping the company turn a profit only months into the drug’s launch.
By Jacob Bell • May 11, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
New Alzheimer's drugs
Alzheimer’s doctors see promise, limits in Lilly’s latest drug data
While some see the new donanemab results as adding to a “watershed moment” in Alzheimer’s research, others say they reinforce the limitations of so-called anti-amyloid therapies.
By Jacob Bell • May 10, 2023 -
Cancer vaccine from BioNTech, Roche shows potential in small study
The companies’ personalized shot generated tumor-specific T cells that researchers hope could prevent pancreatic cancer from returning after surgery.
By Jonathan Gardner • May 10, 2023 -
Gilead acquires San Diego startup for early-stage cancer, immune drugs
The buyout of privately held XinThera continues a string of recent acquisitions of smaller biotechs by Gilead and hands it a group of drug prospects that could begin clinical testing later this year.
By Ned Pagliarulo • May 9, 2023 -
Roche pays China-based biotech $70M for a new HER2 drug
The drug, currently in Phase 1, is an oral medicine designed to cross the blood-brain barrier and better treat HER2 tumors that have spread to the brain.
By Jonathan Gardner • May 9, 2023 -
EQRx resets strategy, abandoning plans to disrupt drug pricing
Launched in 2020 with a "radical" vision, EQRx ran into roadblocks that stymied its efforts to develop new medicines and undercut competitors on price.
By Ned Pagliarulo • May 8, 2023 -
Recursion to acquire two Canadian drug discovery startups
The company agreed to buy Cyclica and Valence after winnowing down a list of more than 100 potential takeover targets, its CEO told BioPharma Dive.
By Gwendolyn Wu • May 8, 2023 -
Former Rubius, Laronde CEO Cagnoni to join Incyte
Six months after being named head of RNA-focused biotech Laronde, Pablo Cagnoni has left the buzzy startup to run Incyte’s R&D work.
By Delilah Alvarado • May 8, 2023 -
Sponsored by OM1
Amplifying lupus disease activity with real-world data and machine learning
Although there is currently no cure, there is great promise in the fight against lupus. Real-world data and artificial intelligence are both critical to the future of clinical research.
By Jessica Probst, MPH, Senior Specialist, Real World Evidence, OM1 • May 8, 2023 -
FibroGen’s anemia pill falls short in blood cancer study
The medicine, available in Europe but rejected two years ago by U.S. regulators, didn’t eliminate the need for blood transfusions among patients with myelodysplastic syndrome.
By Jonathan Gardner • May 5, 2023 -
Convergent raises $90M to develop its radiopharmaceutical for prostate cancer
The biotech is the latest startup to capitalize on rising investor interest in targeted drugs that use radioisotopes to destroy tumors.
By Delilah Alvarado • May 5, 2023 -
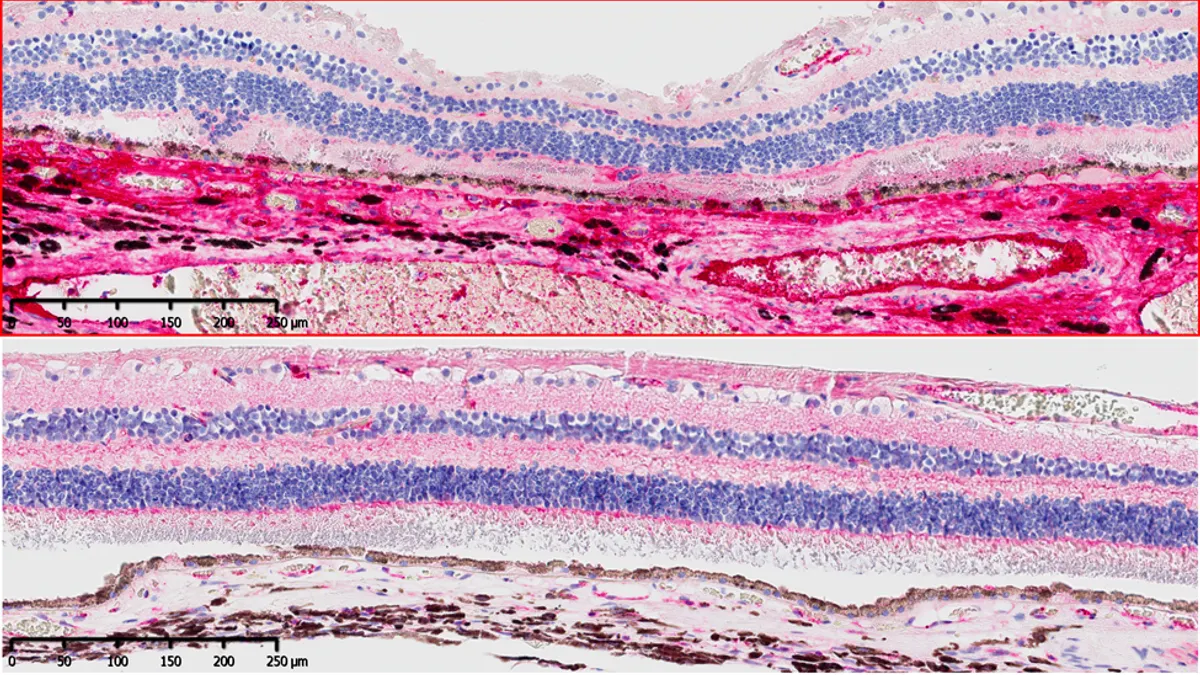
Apellis reports strong start for new vision loss drug
Higher-than-expected sales of the geographic atrophy therapy Syfovre pushed shares in Apellis higher, and could add to speculation about a takeover.
By Ned Pagliarulo • May 5, 2023 -
Acelyrin follows Kenvue with largest IPO for a biotech startup since 2021
The $540 million offering adds to evidence suggesting young drugmakers may need to be well into clinical testing before going public in the current market.
By Gwendolyn Wu • Updated May 8, 2023 -
Lilly’s new Alzheimer’s data may both help and hinder rival Biogen
The success of Lilly’s donanemab in slowing Alzheimer’s progression should reinforce the potential of Leqembi — a similar, recently approved medicine from Eisai and Biogen — but may also make for stiff competition.
By Jacob Bell • May 3, 2023 -
Magenta agrees to reverse merger with Dianthus after setbacks
The deal comes months after Magenta reported a clinical trial setback, began a strategic review of its business and laid off most of its workforce.
By Christopher Newman • May 3, 2023 -
Cerevel hires former Translate Bio head Renaud as new CEO
Ron Renaud, who previously ran and sold two other biotechnology companies, will step in for Tony Coles to lead Cerevel, a Pfizer spinout developing a closely watched schizophrenia drug.
By Kristin Jensen • May 3, 2023 -
Travere set back by study failure of kidney disease drug
Shares in the biotech, formerly Retrophin, sank after the company disclosed its newly approved drug sparsentan failed a study meant to expand its use.
By Ned Pagliarulo • May 2, 2023 -
Ovid partners with Waksal startup to develop drugs for rare brain disorders
Founded by Sam Waksal, Graviton Biosciences is developing drugs that block an enzyme called ROCK2, which drew the interest of neurology-focused Ovid.
By Gwendolyn Wu • May 1, 2023 -
Initial launches with $75M and a new idea for targeting problematic proteins
The company was co-founded by, among others, Jamie Cate, husband of CRISPR pioneer Jennifer Doudna, and Kevan Shokat, a chemical biologist whose previous work helped lead to KRAS-targeting cancer drugs.
By Ned Pagliarulo • May 1, 2023 -
Sanofi strikes a $150M deal for Maze’s Pompe disease drug
The cash influx extends Maze’s financial runway into 2025, giving the South San Francisco company time to advance other drug candidates into testing.
By Gwendolyn Wu • May 1, 2023 -
Sangamo to lay off 120 as it pares pipeline
The cell and gene therapy developer will also cut back on manufacturing and research in California as it redirects resources to three priority programs.
By Ned Pagliarulo • April 27, 2023 -
A startup taking a different approach to Alzheimer’s gets fresh funds, Lilly backing
Therini Bio is developing drugs that target fibrin, a protein involved in wound healing that the company thinks could also play a role in certain neurological and eye diseases.
By Gwendolyn Wu • April 27, 2023 -
Orbital raises $270 million in biotech’s largest Series A round this year
Founded by Howard Chang, John Maraganore and others, the RNA-focused startup drew investment from a large group of blue-chip venture firms.
By Gwendolyn Wu • April 26, 2023 -
ALS drug development
FDA approves new ALS medicine in precedent-setting decision
Biogen’s drug failed the key study meant to show it can slow the nerve-destroying disease, but the drug’s effect on a protein of interest led the FDA to conditionally clear it for market.
By Jacob Bell • Updated April 25, 2023