Biotech: Page 58
-
Roche, Biogen sign royalty deal for late-stage lymphoma drug
Roche will pay royalties to Biogen on glofitimab, part of a long-standing collaboration between the two companies that’s resulted in Rituxan and Ocrevus.
By Jonathan Gardner • Updated Dec. 26, 2022 -
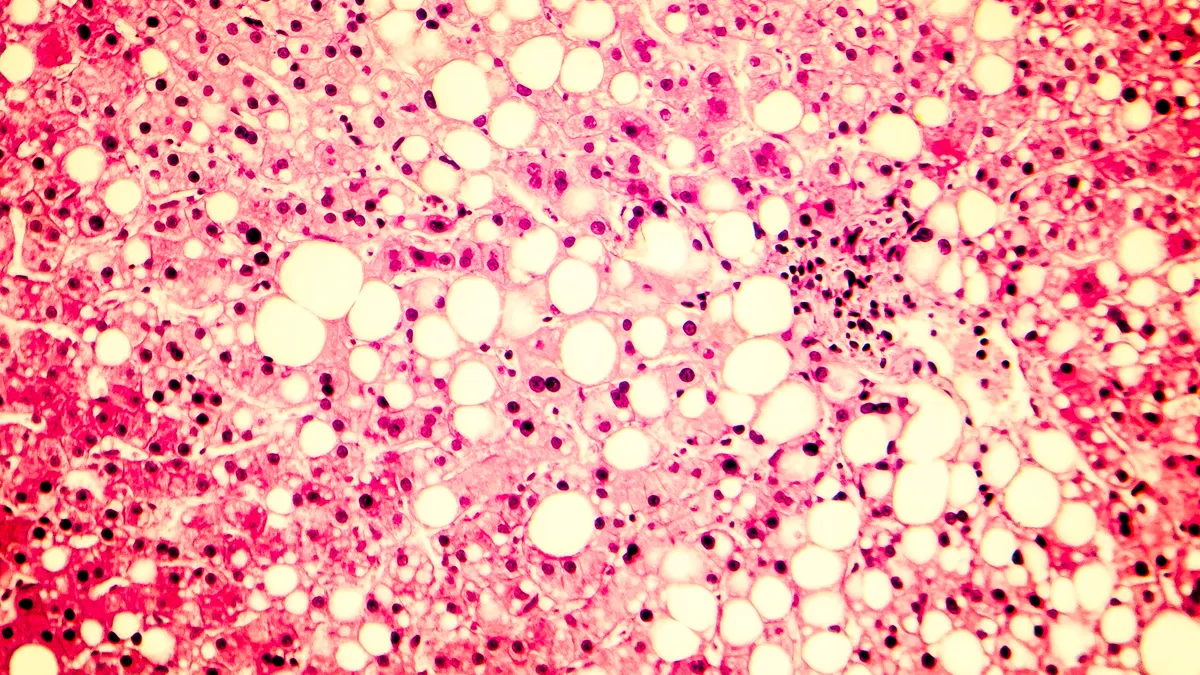
Madrigal shares triple on positive NASH study results
The company said its experimental drug helped patients with the fatty liver disease while also improving fibrosis. One analyst called the readout a “major win” for Madrigal and the NASH field.
By Jacob Bell • Dec. 19, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
Q&A // Emerging biotech
Nimbus CEO Jeb Keiper on negotiating a $4B deal and building an unorthodox biotech startup
Days after inking one of the largest deals for an unapproved drug, the CEO spoke with BioPharma Dive about the company’s unusual origins and decision to remain private — for now.
By Ben Fidler • Dec. 16, 2022 -
Sio Gene Therapies to dissolve after years of setbacks
Having failed to find a buyer or potential partner, the biotech company has opted instead to shut down and liquidate its assets.
By Delilah Alvarado • Dec. 15, 2022 -
A San Diego startup sees covalent biologics as the next frontier in cancer drugs
Enlaza claims to be the first biotech to build a pipeline of protein drugs that can grab their target via covalent binding, a twist on previous drugmaking methods.
By Gwendolyn Wu • Dec. 15, 2022 -
Deep Dive // ALS drug development
For ALS patients, doctors, a new medicine reignites concerns about healthcare access
Relyvrio, a drug developed by Amylyx Pharmaceuticals, is in high demand in ALS clinics across the U.S. Though some patients are already getting it, insurance coverage and out-of-pocket costs remain a source of anxiety.
By Jacob Bell , Shaun Lucas • Dec. 15, 2022 -
Cytokinetics heart drug voted down by FDA panel
Following years of development, the biotech’s heart failure drug now faces another hurdle in its regulatory path forward, after a group of FDA advisers took issue with its trial evidence.
By Delilah Alvarado • Dec. 14, 2022 -
GSK gives Wave a lift with genetic medicine deal
A four-year agreement between the companies comes with $170 million in cash and equity for Wave, which in recent years has dealt with clinical setbacks.
By Kristin Jensen • Dec. 14, 2022 -
Mirati’s KRAS-blocking cancer drug approved by FDA
Cleared for a certain kind of mutated lung cancer, the medicine will challenge Amgen’s similarly acting treatment Lumakras.
By Ned Pagliarulo • Dec. 13, 2022 -
Moderna vaccine succeeds in early-stage skin cancer study with Merck’s Keytruda
The positive data are the most significant findings for the mRNA developer’s pipeline of experimental treatments outside of infectious diseases, where it has reaped billions from COVID-19 vaccine sales.
By Jonathan Gardner • Dec. 13, 2022 -
Black Diamond spins out new biotech to develop cancer drugs
Months after restructuring and trimming its workforce, the company has teamed up with its original investors to form an antibody drug developer called Launchpad Therapeutics.
By Delilah Alvarado • Dec. 13, 2022 -
Q&A
Flagship’s Lovisa Afzelius on creating a successful biotech and a ‘very different’ startup market
Even with a shaky market for drug startups, the Apriori CEO and Alltrna cofounder said Flagship will continue its approach of betting on cutting-edge, and unproven, science.
By Gwendolyn Wu • Dec. 13, 2022 -
Roche’s hemophilia gene therapy holds steady with longer-term data
Roche’s subsidiary Spark has offered few updates on its hemophilia A treatment since being acquired in 2019. New data at ASH show the therapy can maintain levels of a key blood-clotting protein for years.
By Jonathan Gardner • Dec. 12, 2022 -
Clovis files for bankruptcy
Perhaps best known for its marketed PARP inhibitor Rubraca, the cancer drugmaker is now in the process of reorganizing its debt and selling off assets.
By Jacob Bell • Dec. 12, 2022 -
Sponsored by Allucent
[Podcast] Thinking Big for Small and Mid-Sized Biotechs
This podcast series explores how to help small and mid-sized companies get innovative treatments to the people who need them.to help small and mid-sized companies get innovative treatments to the people who need them.
By BioPharma Dive's studioID • Updated Dec. 15, 2022 -

 Retrieved from Istock.
Retrieved from Istock. Sponsored by Veradigm
Sponsored by VeradigmBiopharma trends leaders should consider for 2023
What healthcare trends biopharma leaders should pay attention to in 2023 and how to set yourself up for success.
By Tom Langan, President and Chief Commercial Officer, Veradigm • Dec. 12, 2022 -
Biotech layoffs claim more jobs at Sensei, Instil, TherapeuticsMD
Cell therapy Instil Bio will cut 60% of its staff and Sensei Bio will reduce its headcount by 40%. TherapeuticsMD, meanwhile, is laying off all its employees days after announcing a deal to sell off its drug portfolio.
By Delilah Alvarado • Dec. 9, 2022 -
Dantari emerges with a new way to make cancer drug conjugates
As the success of drugs like Enhertu catalyzes new investment in antibody-drug conjugates, the California startup is launching with plans to develop more potent versions of the targeted cancer medicines.
By Gwendolyn Wu • Dec. 8, 2022 -
Immune drug developer Apogee emerges as first spinout of biotech Paragon
The new biotech launches with $169 million in venture funding and plans to advance its first drug into clinical testing next year.
By Delilah Alvarado • Dec. 7, 2022 -
MEI, Kyowa stop lymphoma drug trials after FDA meeting
The decision not to run a Phase 3 trial is the latest fallout from U.S. regulators’ recent moves to closely evaluate a class of drugs called PI3 kinase inhibitors.
By Jonathan Gardner • Dec. 6, 2022 -
Karuna switches CEOs ahead of FDA filing for schizophrenia drug
Former Allergan commercial executive Bill Meury will take over for longtime CEO Steve Paul as the company prepares to seek U.S. approval of the treatment, known as KarXT, next year.
By Christopher Newman • Dec. 6, 2022 -
Star Therapeutics launches Vega, growing its galaxy of drug startups
The second startup to emerge from Star’s “hub-and-spoke” model aims to develop a Hemlibra-like antibody drug for the blood clotting disorder von Willebrand disease.
By Gwendolyn Wu • Dec. 6, 2022 -
With $81M, Entact Bio takes a new approach to fixing helpful proteins
Entact Bio aims to develop medicines that can enhance helpful proteins, rather than block harmful ones, a twist on the usual drugmaking playbook.
By Gwendolyn Wu • Dec. 6, 2022 -
Q&A // Emerging biotech
Biotech veteran Jeff Jonas on leaving Sage, guiding new biotechs and his ‘personal odyssey’
In an interview with BioPharma Dive, the longtime industry executive discussed becoming a biotech investor and his plans for Abio-X, a new incubator with $150 million to spend on startups.
By Ben Fidler • Dec. 6, 2022 -
TherapeuticsMD to offload drugs to Australia’s Mayne after failed buyout
After a private equity takeover unraveled this summer, the women’s health company is selling its drug portfolio to Mayne Pharma in a $153 million deal.
By Delilah Alvarado • Dec. 5, 2022