Biotech: Page 61
-
Vaxcyte rides investor enthusiasm for early pneumococcal vaccine data
Encouraging study results for a vaccine with broader coverage than Pfizer’s Prevnar 20 sent Vaxcyte’s valuation soaring and could make the company a potential takeover target.
By Jonathan Gardner • Oct. 24, 2022 -
Sponsored by Aldevron
Simplify IP for your gene therapy with OTS backbones, plasmids and enzymes
Read how IP-simplified products can transform your speed to market without needing to reassess operations midway through to commercialization.
Oct. 24, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
Gilead, working to improve cancer cell therapy, partners with California startup
Synthetic biology technology developed by the startup, Refuge Biotechnologies, could help Gilead produce safer and more effective CAR-T treatments for certain blood cancers.
By Ned Pagliarulo • Oct. 20, 2022 -
AbbVie to buy UK biotech DJS for $255M
The deal gives AbbVie an experimental medicine for idiopathic pulmonary fibrosis, as well as a platform for designing antibody drugs that can target proteins known as GPCRs.
By Ned Pagliarulo • Oct. 20, 2022 -
Zymeworks strikes deal with Jazz as HER2 drug developers adjust their plans
The high bar set by AstraZeneca and Daiichi Sankyo’s HER2-targeting drug Enhertu appears to have influenced Zymeworks’ decision, while leading to a restructuring at Ambrx Biopharma.
By Kristin Jensen • Oct. 19, 2022 -
A startup plans an IPO to give a shelved Lilly drug another shot
Acrivon Therapeutics is looking to fund a development approach it claims could improve the prospects of a cancer drug Lilly scrapped after testing it in a handful of trials.
By Gwendolyn Wu • Oct. 19, 2022 -
State of Play
‘In vivo’ cell therapy: expanding beyond CAR-T
At least five startups have emerged with new ways to genetically modify immune cells within the body, an approach that, if successful, could widen the field of CAR-T treatment.
By Ben Fidler • Oct. 18, 2022 -
Treeline Bio deepens investor roots with fresh funding for cancer drug research
Led by former Loxo and Novartis executives, Treeline has divulged few details about its research, even as it’s raised nearly half a billion dollars and grown to over 130 employees.
By Gwendolyn Wu • Oct. 17, 2022 -
Biogen expects longer wait on ALS drug decision from FDA
The agency has requested more information about tofersen, a closely watched medicine that Biogen is seeking approval of despite a failed Phase 3 trial.
By Jacob Bell • Oct. 17, 2022 -

Mateusz/Shutterstock.com
 Sponsored by Emergent Biosolutions
Sponsored by Emergent BiosolutionsThe demand for nanoparticles in drug formulation is rising
Collaborating with an experienced partner, such as a Contract Development & Manufacturing Organization (CDMO), could prove to be invaluable in developing nano-based systems.
By Kate Silver • Oct. 17, 2022 -
Covalent drugs take the stage — again — in Atlas Venture’s latest startup
A decade after Celgene bought the Atlas-backed Avila Therapeutics, the venture firm is building Matchpoint Therapeutics to develop covalent medicines for autoimmune diseases.
By Gwendolyn Wu • Oct. 14, 2022 -
Odyssey recruits new investors to fund precision drug research
A $168 million Series B round brings Odyssey’s total funding to date near $400 million, helping the startup support a growing pipeline of cancer and immune disease drug candidates.
By Ned Pagliarulo • Oct. 13, 2022 -
Nimbus partners with Eli Lilly on metabolic disease research
While Nimbus has drawn attention for its work on a TYK2 inhibitor, Lilly is interested in the company’s research around an enzyme called AMPK that’s seen as a useful, but hard-to-reach drug target.
By Delilah Alvarado • Oct. 12, 2022 -
Merck pays $250M to license Moderna cancer vaccine
The drugmaker’s decision to grab rights to the shot deepens its ties with the COVID-19 vaccine developer and comes weeks before a key data release.
By Kristin Jensen • Oct. 12, 2022 -
Vertex, after setbacks, moves forward with second-generation rare disease drug
The biotech is starting a trial of a new treatment for alpha-1 antitrypsin deficiency following disappointing study results for two earlier compounds.
By Ned Pagliarulo • Oct. 11, 2022 -
BIO’s McMurry-Heath steps down as head of biotech lobby
Her exit from the role follows reported disagreements over the group’s direction. Former GlycoMimetics CEO Rachel King will serve as interim CEO during the search for a successor.
By Ned Pagliarulo • Oct. 11, 2022 -
A young biotech’s shares soar on early psoriasis drug results
Study data from Dice Therapeutics, which went public just a year ago, suggest its experimental pill has a chance to challenge marketed drugs from Amgen and Bristol Myers.
By Jonathan Gardner • Oct. 11, 2022 -
Brain disease startup Neumora draws more funding for ambitious research plan
Launched last year to change how drugs for brain diseases are made, the biotech has now raised about $650 million to build a pipeline that includes two clinical-stage medicines.
By Gwendolyn Wu • Oct. 11, 2022 -
State of Play
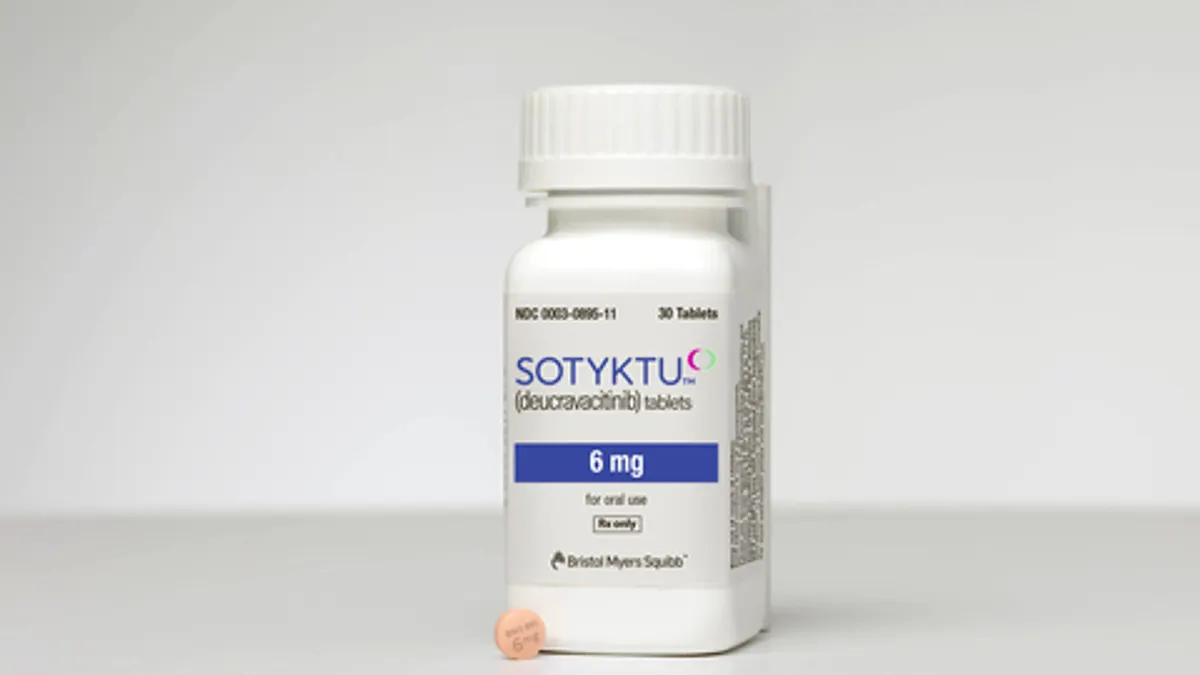
New TYK2 inhibitors: a growing race to top Bristol Myers
The pharmaceutical company’s psoriasis drug Sotyktu was the first of its kind to win approval. A group of well-funded startups think they can do better.
By Gwendolyn Wu • Oct. 11, 2022 -
PureTech, Nektar end talks of combination
Days after acknowledging that discussions were taking place, the two companies have called off further negotiations over a possible deal, leaving Nektar’s future again uncertain.
By Jonathan Gardner • Updated Oct. 11, 2022 -
BioMarin to lay off about 120 employees as part of restructuring plan
BioMarin said the reorganization is meant to reflect its transition to a large-scale biopharmaceutical company with a more diversified portfolio.
By Jacob Bell • Oct. 7, 2022 -
A Versant-backed biotech emerges to take on ‘overlooked’ cancer targets
Nested Therapeutics touts a roster of scientific leaders, including Kevan Shokat, whose work drugging KRAS — a cancer-related gene once thought to be undruggable — helped lead to Amgen’s Lumakras.
By Jacob Bell • Oct. 6, 2022 -
Nobel Prize in chemistry awarded to biotech founder Bertozzi, two others
Carolyn Bertozzi, a Stanford scientist and co-founder of several biotechs, helped advance "click chemistry" — a concept pioneered by Morten Meldal and Barry Sharpless, with whom she shares this year's prize.
By Delilah Alvarado • Oct. 5, 2022 -
Biohaven starts life as new company following Pfizer buyout
Spun out as part of Pfizer’s $11.6 billion acquisition, the biotech retains a pipeline of experimental neuroscience drugs and holds $258 million in cash.
By Ned Pagliarulo • Oct. 5, 2022 -
KalVista stops work on rare disease drug over safety concerns
The biotech terminated a Phase 2 study after treatment led to serious liver enzyme elevations in multiple patients with hereditary angioedema.
By Ned Pagliarulo • Oct. 4, 2022