Biotech: Page 65
-
Q&A
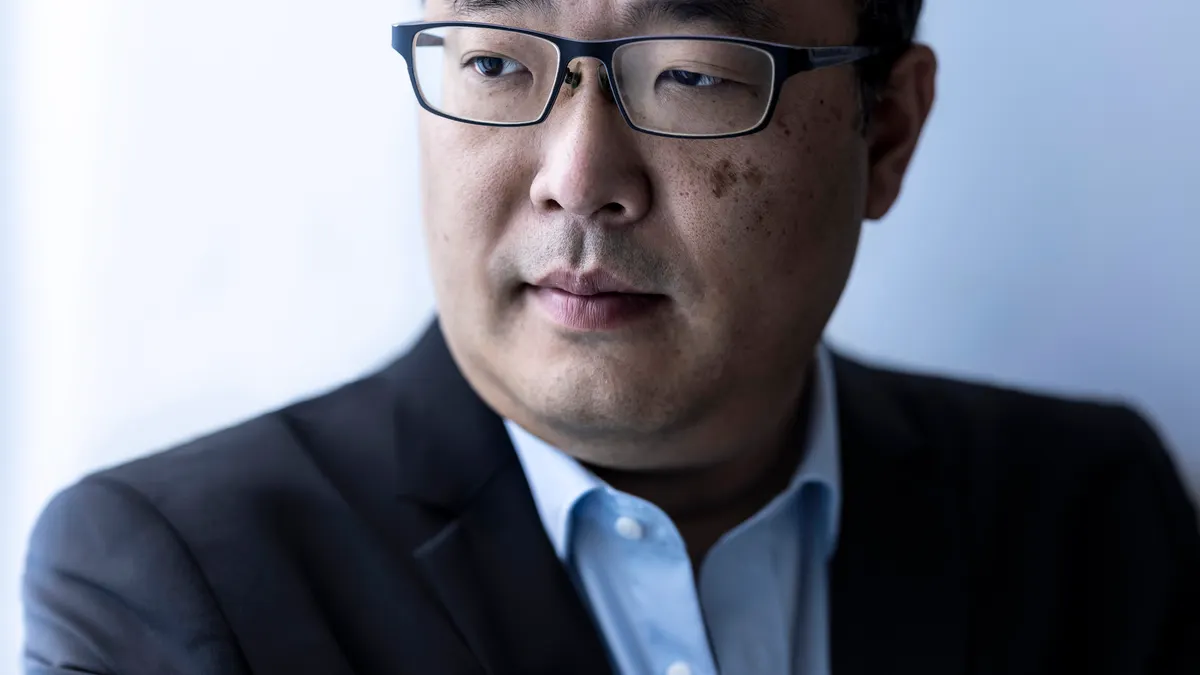
Strand CEO on founder-led biotech, venture capital and the market’s retreat
Jake Becraft, founder and current chief of mRNA startup Strand Therapeutics, argues biotech would be healthier if more scientists and researchers lead the companies they start.
By Ned Pagliarulo • Aug. 2, 2022 -
How Belite Bio, a small, under-the-radar drugmaker, emerged as one of biotech’s best performing IPOs
Belite's shares have risen nearly 500% from their IPO price at a time when many other newly public biotech companies are struggling to hold their value.
By Delilah Alvarado • Updated Aug. 2, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
Nuvation to cut workforce by 35% as it scraps lead drug program
The company, which is led by former Medivation executives and focuses on cancer research, said the shake-up is in response to an “internal risk-benefit analysis” that raised doubts about its most advanced program.
By Jacob Bell • Aug. 1, 2022 -
Concert to seek approval for hair loss drug after second study success
The biotech plans to file an application with the FDA in the first half of next year for clearance in alopecia areata, potentially positioning it to compete with Eli Lilly and Pfizer for market share.
By Ned Pagliarulo • Aug. 1, 2022 -
Sarepta to ask FDA for accelerated approval of Duchenne gene therapy
After discussions with the FDA, the biotech aims to submit an application this fall — sooner than expected and ahead of a Phase 3 study that’s now ongoing.
By Jonathan Gardner • July 29, 2022 -
Alnylam reveals longer wait for anticipated drug trial results
Important data from the APOLLO study of the biotech's drug Onpattro are due before the end of August, Alnylam said Thursday.
By Ned Pagliarulo • July 28, 2022 -
Novartis-linked startup launches with technology designed to remove ‘destroy’ tags on helpful proteins
Vicinitas and its protein-stabilizing research have attracted $65 million from a group of investors that includes a16z, Deerfield and GV.
By Jacob Bell • July 28, 2022 -
Versant partners with AbCellera to arm startups with better antibodies
The companies pitch their collaboration as a way to give young drugmakers backed by Versant a head start. “Our business is advanced by great ideas coming into the funnel,” said AbCellera’s CEO.
By Ned Pagliarulo • July 27, 2022 -
BridgeBio advances drug for dwarfism after study data
The highest tested dose of BridgeBio’s drug helped the bones of children with achondroplasia grow faster, leading the company to expand study enrollment.
By Ned Pagliarulo • July 26, 2022 -
Seagen, Astellas claim positive results in study key to cancer drug's success
The mid-stage trial tested the companies’ drug Padcev with Merck’s Keytruda in first-line bladder cancer. The data are reportedly a factor in Merck’s talks with Seagen over a potential buyout.
By Ned Pagliarulo • July 26, 2022 -
FDA, in another test of its flexibility, agrees to review Biogen's closely watched ALS drug
Known as tofersen, the drug failed in the main study being used to support its approval. But an apparent effect on a protein of interest in ALS research has Biogen convinced the treatment will pass muster with regulators.
By Jacob Bell • July 26, 2022 -
State of Play
Epigenetic editing: a tunable CRISPR alternative
Three startups have recently emerged with plans to edit the epigenome rather than DNA directly. Here’s why that matters and what they aim to do.
By Ben Fidler • July 26, 2022 -
Deep Dive // Emerging biotech
‘Flat is the new up’: After biotech correction, venture investors turn to safer bets
New companies are being built more carefully and platform technologies are less in vouge as the downturn's effects ripple through the private sector.
By Ben Fidler • July 26, 2022 -
China-based biotech licenses Sanofi's RNA drug technology
The Lilly-backed Rona Therapeutics gains rights to a platform for small interfering RNA, along with a slate of preclinical candidates aimed at targets in the liver and other tissues.
By Ned Pagliarulo • Updated July 25, 2022 -
Astellas to bring research units under one roof in new West Coast center
The pharma company plans to spend $70 million on the South San Francisco facility, which will house West Coast employees of Astellas Gene Therapies.
By Ned Pagliarulo • July 22, 2022 -
Assembly Bio scraps hepatitis B drug, cuts staff after clinical setback
Two top executives will depart in a restructuring that will reduce the California biotech’s staff by 30% and prioritize a pair of preclinical research programs.
By Kristin Jensen • July 21, 2022 -
Vertex, Verve team up to develop a gene editing drug for liver disease
The deal, which is worth $60 million upfront, broadens Vertex’s reach into gene editing and expands Verve’s research beyond heart disease.
By Ben Fidler • July 20, 2022 -
With Aduhelm sidelined, Wall Street turns to Biogen's next Alzheimer's drug
On the company’s latest earnings call, analysts pressed for more details about the drug, called lecanemab, including its odds for approval and how that could reshape Biogen.
By Jacob Bell • July 20, 2022 -
Inovio to lay off 18% of staff after COVID shot struggles
The cuts are meant to conserve cash as the biotech attempts to position its coronavirus vaccine as a potential booster.
By Kristin Jensen • July 20, 2022 -
Roche digs deeper into gene therapy for the eye
The Swiss drugmaker, which already owns rights to a marketed gene therapy for inherited vision loss, will work with startup Avista Therapeutics to develop better delivery tools for the complex treatments.
By Jonathan Gardner • July 20, 2022 -
Biotech downturn hits another startup as Pact Pharma to lay off nearly 100 employees
The cell therapy developer, which was formed in early 2017 by a group that includes Nobel Prize winner David Baltimore, is one of dozens of biotechs to cut staff this year.
By Delilah Alvarado • July 19, 2022 -
FDA approves new vitiligo treatment, bolstering Incyte's dermatology business
The cream, known as Opzelura, is now the only treatment approved in the U.S. to repigment skin in patients with the most common form of vitiligo.
By Jacob Bell • July 19, 2022 -
Sponsored by OM1
Finding hard-to-find patients: Integrating real-world data and AI
Better understanding patient cohorts can shed light on outcomes, but what if coding doesn’t exist?
By Joseph Zabinski, PhD Senior Director, AI and Precision Medicine OM1 • July 18, 2022 -
Novo strikes $110M deal for regulatory fast pass
The Danish drugmaker will buy a priority review voucher from Marinus Pharmaceuticals, the latest in a string of similarly priced purchases.
By Ned Pagliarulo • July 15, 2022 -
CytomX lays off staff, joining growing list of biotechs restructuring
A shaky biotech market has forced another drugmaker to adjust, with CytomX revealing plans to reduce its workforce by 40%.
By Delilah Alvarado • July 14, 2022