Manufacturing: Page 6
-
FDA holds back Alvotech’s Humira biosimilar over manufacturing issues
The company received a complete response letter, but still expects to win approval of the copycat drug in time to launch it in the U.S. next year.
By Jonathan Gardner • Sept. 6, 2022 -
‘Treg’ startup Sonoma expands with plans for manufacturing plant
The biotech, launched by immunologist Jeffrey Bluestone, is developing regulatory T cell therapies for autoimmune and inflammatory diseases.
By Ned Pagliarulo • Aug. 24, 2022 -
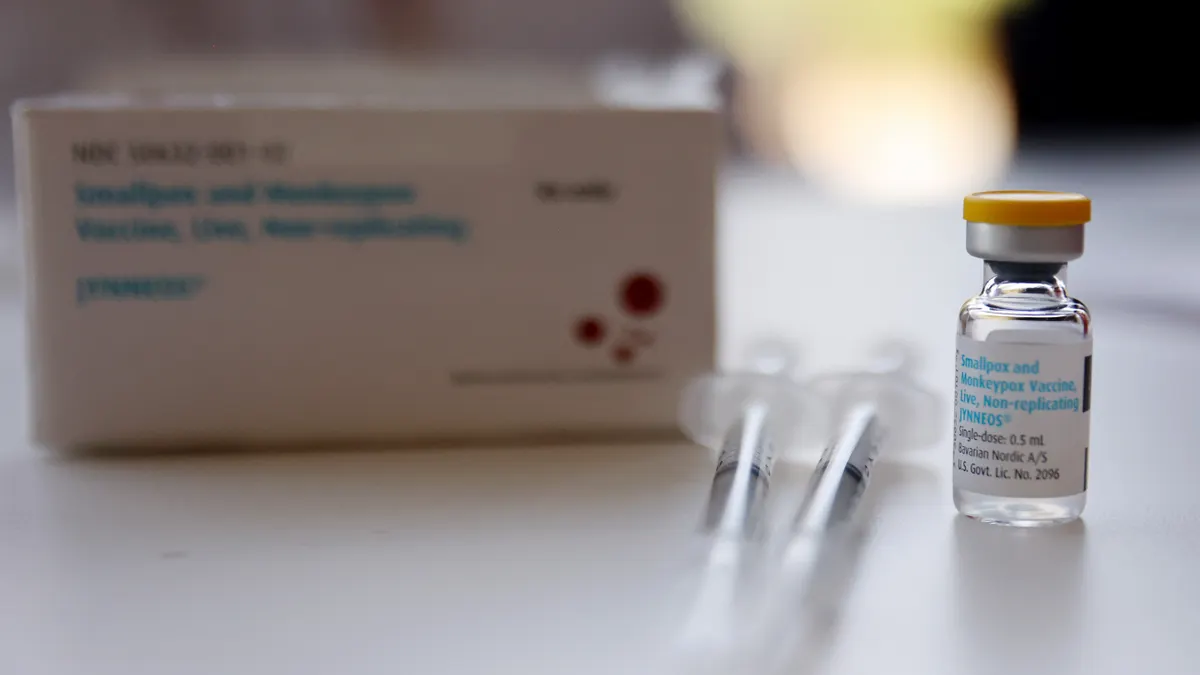
US speeds rollout of monkeypox vaccine, treatment
The Biden administration next week will provide 1.8 million doses of the Jynneos vaccine and 50,000 courses of antiviral Tpoxx to states and local governments.
By Christopher Newman • Aug. 19, 2022 -
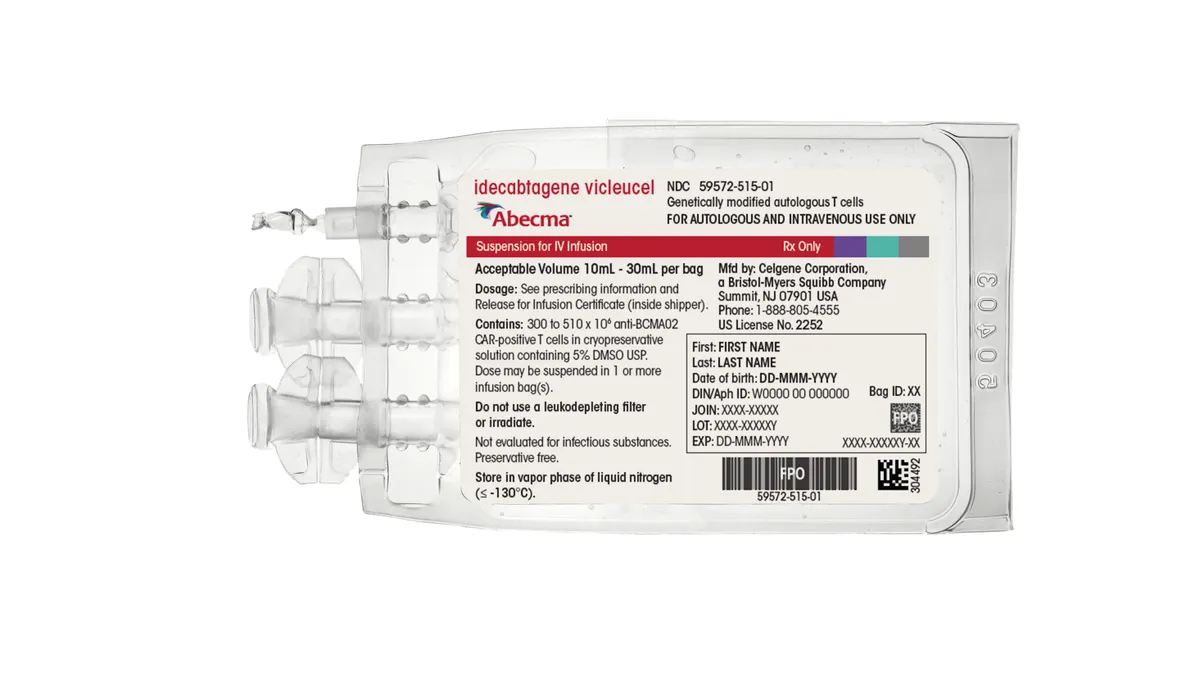
Bristol Myers CAR-T sales rise despite production problems
Demand has increased faster than Bristol Myers can make Abecma, although the company said supply is improving. A manufacturing issue for Breyanzi, meanwhile, impacted sales.
By Ned Pagliarulo • July 27, 2022 -
California to manufacture its own insulin, governor says
Gov. Gavin Newsom announced a $100 million budget to create a production facility and to develop affordable insulin products, saying the medicines’ high cost “epitomizes market failures.”
By Susan Kelly • July 11, 2022 -
Resilience, a well-funded manufacturing startup, joins with the Parker Institute to make cancer drugs
The unusual biotech, which just raised $625 million earlier this week, will work with the immunotherapy center under a five-year agreement to create and spin off new companies.
By Kristin Jensen • June 9, 2022 -
Astellas, despite recent setbacks, opens $100M gene therapy plant
The opening of the plant, which could create more than 200 new jobs, comes on the heels of several significant issues with the company’s most advanced gene therapy program.
By Jacob Bell • June 8, 2022 -
Pfizer to invest $120M, add jobs in Paxlovid production push
After initially struggling to meet demand for Paxlovid, Pfizer is putting significant resources toward expanding an important manufacturing plant in Michigan.
By Delilah Alvarado • June 7, 2022 -
Sponsored by Stevanato Group
Why choosing the right primary container is crucial
New mRNA technology: identifying the right drug containment solution is more important than ever.
May 16, 2022 -
Novartis suspends production of two radiopharmaceutical drugs over quality concerns
Due to potential issues in manufacturing, the pharma is halting deliveries of two cancer drugs, Lutathera and Pluvicto, in the U.S. and Canada, a setback for a business in which the company has invested heavily.
By Ned Pagliarulo • May 6, 2022 -
Seagen to expand, add jobs with new cancer drug factory
While some biotechs are restructuring amid a market downturn, SeaGen plans to add 200 positions through a large manufacturing plant that should be operational in 2024.
By Jacob Bell • April 21, 2022 -
Gilead gets cell therapy boost with FDA clearance of Maryland factory
The new site in Maryland will be used to produce Yescarta, which this month won an expanded approval that substantially widens its market.
By Ned Pagliarulo • April 19, 2022 -
Sanofi API spinout takes step toward market listing
French regulators have approved plans by Sanofi to list the new EUROAPI business on Euronext Paris beginning May 6.
By Ned Pagliarulo • April 1, 2022 -
Novartis turns cell therapy skill into contract manufacturing deal
In an unusual arrangement, the Swiss pharma will make Carisma Therapeutics' experimental cell therapy at its Morris Plains factory, part of its plans to build up a CMO business.
By Ned Pagliarulo • March 10, 2022 -
Novo sees fast growth ahead of obesity drugs despite production hurdles
The Danish drugmaker aims to triple sales of its weight loss treatments Wegovy and Saxenda by 2025. But first, Novo needs to resolve manufacturing constraints that have slowed the former drug's launch.
By Ned Pagliarulo • March 4, 2022 -
Atara sells cell therapy plant to Fujifilm for $100M
The site, which specializes in manufacturing off-the-shelf immunotherapies, was once a key asset for Atara. New owner Fujifilm will still supply the biotech under a long-term agreement.
By Ned Pagliarulo • Jan. 27, 2022 -
Sponsored by Nuvolo
3 ways an IWMS can help streamline your manufacturing operations
Manage, maintain and optimize all the tasks, duties and goals that fall under the umbrella of your workplace.
Jan. 24, 2022 -
Generic manufacturers sign pact to supply Merck COVID pill to lower-income countries
The licenses will help make molnupiravir available to people in more than 100 countries, giving public health authorities a needed tool despite the drug's limited effectiveness.
By Jonathan Gardner • Jan. 20, 2022 -
German Merck buys manufacturer of key component in mRNA medicines
Merck KGaA is buying Indianapolis-based Exelead, a specialist in the lipid nanoparticles used to ferry mRNA into the body, for $780 million in cash.
By Ned Pagliarulo • Jan. 6, 2022 -
Pfizer opens gene therapy plant in $800M North Carolina expansion
The new facility cost nearly $70 million to build, and is part of a major push by the pharma giant to become a leader in genetic medicine manufacturing.
By Kristin Jensen • Dec. 16, 2021 -
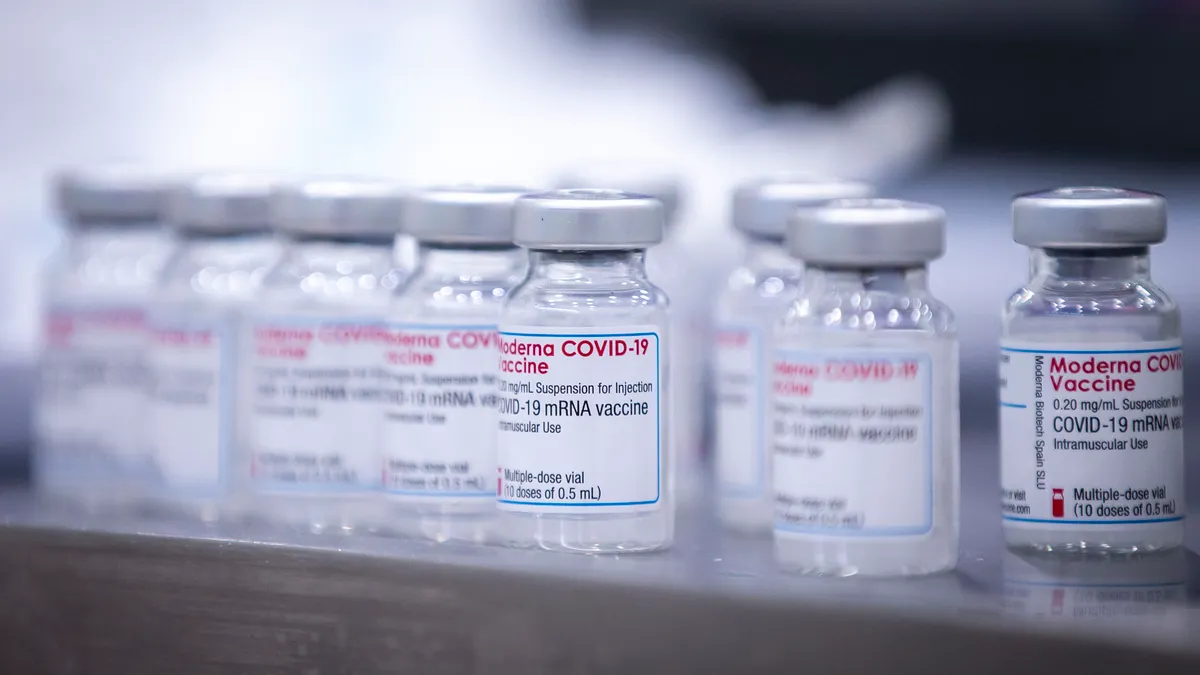
Moderna cuts vaccine sales, delivery targets for 2021
The biotech now expects to deliver between 700 million and 800 million doses this year, down from a previous forecast of 800 million to 1 billion.
By Ned Pagliarulo • Nov. 4, 2021 -
Gates Foundation pledges $120M to bring Merck COVID-19 drug to lower-income countries
Anticipating regulatory clearance of the pill, the group aims to speed access by investing in the manufacturing of generic versions of the drug.
By Kristin Jensen • Oct. 20, 2021 -
Sponsored by Vetter Pharma
Emerging therapeutic oligonucleotides – analytical challenges accepted
A relatively new class of parenteral drug products on the market - therapeutic oligonucleotides – poses new challenges to the pharmaceutical manufacturing process.
By Alexandra H. Heussner, Ph. D., Laboratory Manager ASL, and Melanie Zerulla-Wernitz, Ph. D., Head of ASL, Development Service, Vetter Pharma-Fertigung GmbH & Co. KG • Oct. 18, 2021 -
Under pressure to share technology, Moderna to build vaccine factory in Africa
The biotech company expects to make its coronavirus vaccine and mRNA shots for other diseases at the planned plant, into which it will invest $500 million.
By Kristin Jensen • Oct. 7, 2021 -
US stocks up on COVID-19 antibody drugs amid shortage concerns
Deals with Regeneron and Eli Lilly will secure nearly 1.8 million additional doses of the companies' respective treatments over the next four months.
By Ned Pagliarulo • Sept. 15, 2021