Marketing: Page 8
-
FDA orders new cancer warnings for CAR-T therapies
The agency later modified its warning for Gilead's Tecartus, tweaking the label's language to reflect the fact that none of T cell malignancies in question had occurred in Tecartus-treated patients.
By Jonathan Gardner • Updated Jan. 24, 2024 -
Biogen CEO sees progress in launch of Alzheimer’s drug Leqembi
At the J.P. Morgan Healthcare Conference Monday, company head Chris Viehbacher said insurance reimbursement is now ‘not an issue’ for treatment.
By Jacob Bell • Jan. 8, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Brian Tucker / BioPharma Dive/BioPharma Dive
Brian Tucker / BioPharma Dive/BioPharma Dive Trendline
TrendlineCommercialization
New drugs for obesity are becoming blockbusters, while Trump administration pressure is reshaping pharma marketing strategies ahead of looming patent cliffs.
By BioPharma Dive staff -
Lilly launches online service for home delivery of weight loss drug
The new program, dubbed LillyDirect, will help patients connect with telehealth providers and deliver medicines like the company’s obesity drug Zepbound.
By Ned Pagliarulo • Jan. 4, 2024 -
Sponsored by Alexander Group
Profitable growth is on the horizon
A return to profitable growth is on the horizon—organizations that take advantage of the following key trends can thrive in 2024 and beyond.
Dec. 11, 2023 -
CVS overhauls how its retail pharmacies charge for prescription drugs
Under the new model, CVS’ pharmacy network will price drugs based on the amount the company paid for them, plus a defined markup and additional pharmacist fee.
By Rebecca Pifer Parduhn • Dec. 5, 2023 -
European regulators want to know more about the risks of GLP-1 drugs
The EMA's safety committee has more questions for makers of the in-demand therapies as it reviews whether the drugs are linked to the risk of suicidal thoughts.
By Jacob Bell • Dec. 1, 2023 -
Cigna, Humana in talks to merge: WSJ
The merger would have major effects for the makeup of the U.S. health insurance industry, and would almost certainly face a regulatory challenge.
By Rebecca Pifer Parduhn • Nov. 29, 2023 -
Medtronic CEO downplays impact of obesity drugs on procedures, devices
Surging demand for GLP-1 agonists has put medtech firms like Medtronic under pressure, even as they argue the hit to their businesses will be minimal.
By Ricky Zipp • Nov. 21, 2023 -
Bristol Myers faces FDA delay on cancer cell therapy decision
The regulator plans to convene an advisory panel to discuss an expanded indication for Abecma, presenting another hurdle for Bristol Myers and partner 2seventy bio.
By Ned Pagliarulo • Nov. 20, 2023 -
New AMA policies target GLP-1 coverage, corporate medicine
The lobbying group urged broader health insurance coverage for treating obesity, including with GLP-1 agonists like Wegovy.
By Susanna Vogel • Nov. 14, 2023 -
Wegovy study details revive debate over GLP-1 impact on devices
Shares in several heart device makers, which have been battered over the perceived impact of obesity medicines, rose this week following AHA data on Novo’s therapy.
By Susan Kelly • Nov. 13, 2023 -
Biogen, Sage set price of postpartum depression pill at $15,900
The price is below what some analysts had predicted and significantly less than $34,000 Sage initially set for its earlier postpartum infusion, Zulresso.
By Ned Pagliarulo • Nov. 7, 2023 -
Sponsored by Viatris
Transforming MS care to meet patient needs
Developing holistic treatment approaches that address patient needs today and in the future.
By Abhijit Barve, MD, PhD, MBA, Chief Medical Officer, Viatris • Nov. 6, 2023 -
Novo, Lilly buoyed by fast-growing GLP-1 drug sales
The rival companies reported strong quarterly sales growth for their latest products, which could face off as weight loss treatments next year.
By Jonathan Gardner • Nov. 2, 2023 -
The obesity drug effect: What medical device executives are saying
Stock selloffs across the medical device sector have erased hundreds of billions of dollars in market value. But medtech executives argue GLP-1 drug demand will complement, not cannibalize, their sales.
By Susan Kelly • Nov. 2, 2023 -
GSK outpaces Pfizer in RSV vaccine market
Sales of GSK’s new RSV shot Arexvy were more than double what Pfizer reported for its rival product, suggesting substantial demand this fall.
By Kristin Jensen • Nov. 1, 2023 -
Sponsored by Veeva
5 things to look for in a marketing analytics partner
Effective marketing measurement requires objectivity, connected data, transparency, a track record of innovation and a proven ability to execute.
Oct. 23, 2023 -
Roche eye drug sales grow as Regeneron plays catch-up
The Swiss pharma reported expanding U.S. market share for its new AMD drug Vabysmo as Regeneron tries to rebound from a now-resolved delay for its high-dose Eylea.
By Jonathan Gardner • Oct. 19, 2023 -
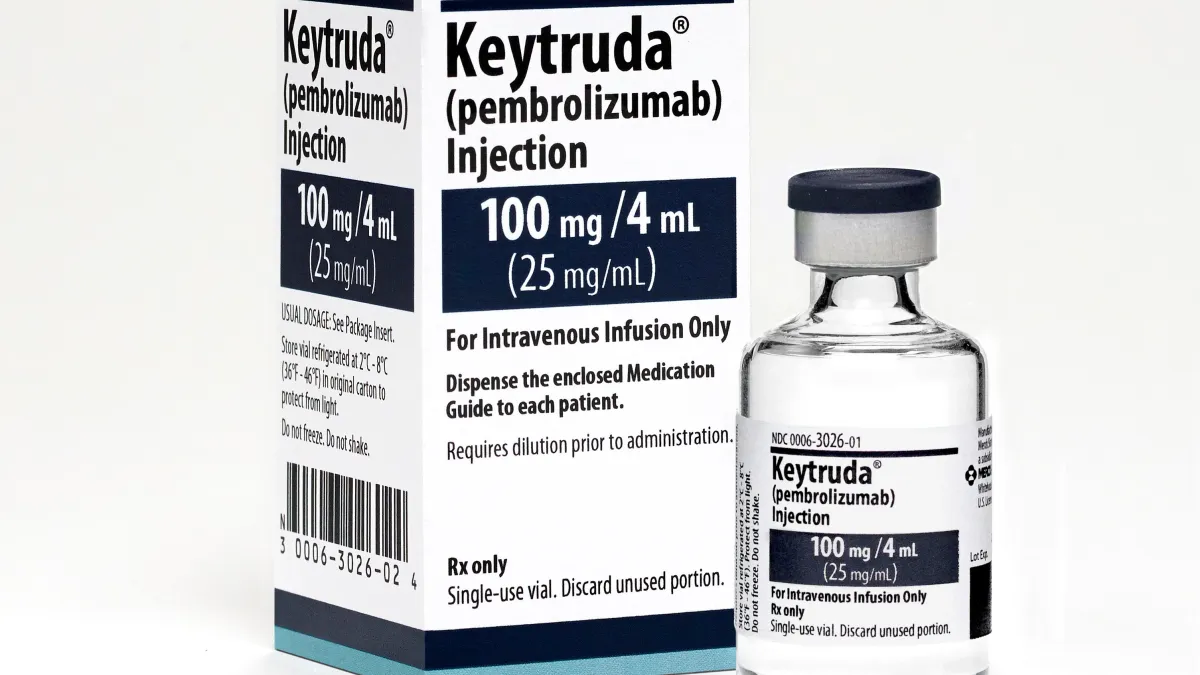
Keytruda gains first approval for pre- and post-surgery use in lung cancer
The Merck drug leads competitors Opdivo and Imfinzi into a new immunotherapy setting, which aims to improve outcomes following the surgical removal of tumors.
By Jonathan Gardner • Oct. 17, 2023 -
Pfizer wins FDA approval for its $7B colitis drug
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
By Jonathan Gardner • Oct. 13, 2023 -
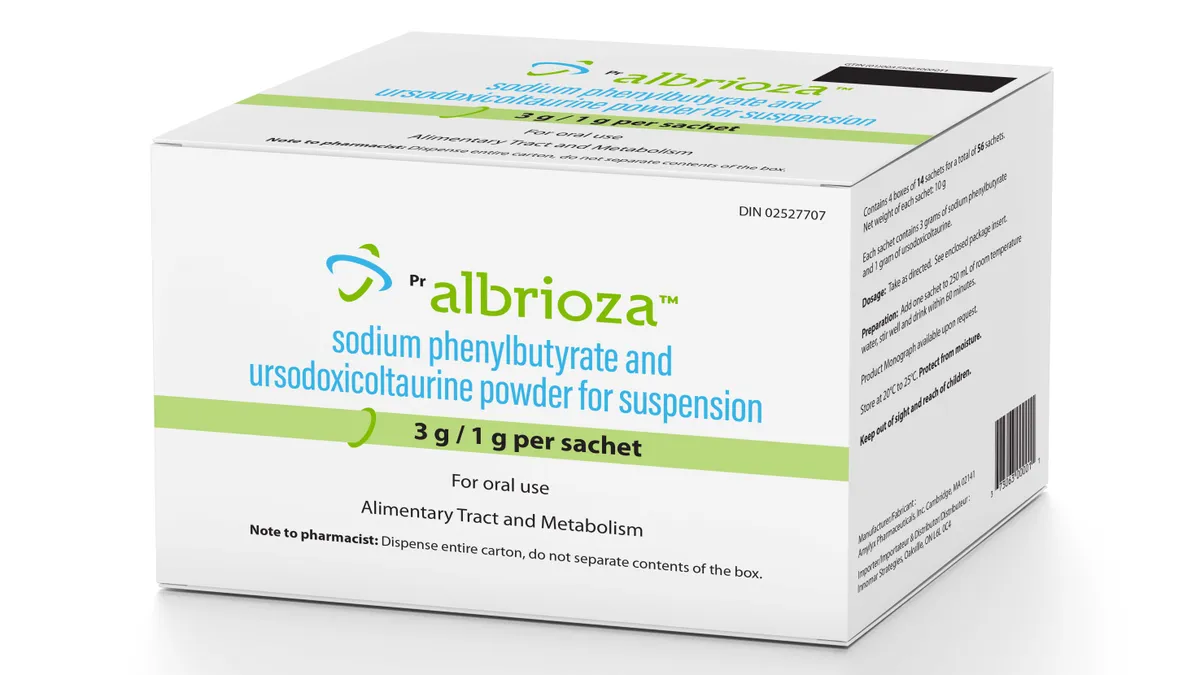
ALS drug development
Amylyx’s ALS drug knocked back again in Europe
Following a re-examination, the EMA again concluded that Albrioza — known as Relyvrio in the U.S. — should not be cleared for the European market.
By Jacob Bell • Oct. 13, 2023 -
GLP-1 drug demand leaves employers in a bind over coverage
Employers face a difficult decision over whether to cover the popular weight loss therapies, which cost between $900 and $1,400 for a typical month’s supply at list price.
By Rebecca Pifer Parduhn • Oct. 13, 2023 -
Apellis sales numbers show steady demand for new eye drug, despite safety worries
The company says Syfovre prescriptions accelerated again in August after a rocky summer of side effect probes.
By Kristin Jensen • Oct. 5, 2023 -
Sponsored by Cognizant
Using AI to create an end-to-end customer experience in pharma
Silos abound in the pharma world, especially when it comes to customer care and call center support. Using AI can change everything, though. Here’s how.
Oct. 2, 2023 -
Eisai’s Alzheimer’s drug Leqembi approved in Japan
Eisai and Biogen’s anti-amyloid drug is still undergoing regulatory reviews in the EU, U.K. and elsewhere.
By Ned Pagliarulo • Sept. 25, 2023