The Latest
-
The top biopharma conferences in 2026
Medical meetings often feature important clinical trial results, making them barometers of biotech and pharma companies’ research progress. Here’s a list of conferences to watch next year.
-
Deep Dive // Emerging biotech
Biotech IPOs are the industry’s lifeblood. Track how they’re performing.
Evommune on Thursday outlined plans for an offering that would support development of two drugs in mid-stage testing for urticarias and atopic dermatitis.
Updated 22 hours ago -
Regeneron, needing a turnaround, gains new use for cancer drug
Libtayo’s latest approval for a type of skin cancer should boost future sales, according to one analyst, but doesn’t have the “broad-reaching implications” for Regeneron's Eylea franchise investors had hoped.
-
 Bhagavatheeshwaran, Govind. (2016). "MRI Scan" [Image]. Retrieved from Flickr.
Bhagavatheeshwaran, Govind. (2016). "MRI Scan" [Image]. Retrieved from Flickr. China competition
China competitionZenas looks to China to stock pipeline with 3 more immune drugs
A potentially $2 billion deal with InnoCare Pharma hands Zenas rights to a BTK inhibitor in late-stage testing for multiple sclerosis and two other immune medicines in earlier development.
-
Deep Dive // Pain drugs
Cancer patients are living longer than ever. Pain drugmakers haven’t kept up.
Decades of slow-moving research, along with broader failures of the healthcare system, have left millions of people in daily pain. Doctors fear that’s bound to continue.
-
Emerging biotech
Arthrosi snags $153M in pursuit of a new gout drug
The company is one of a handful developing a more “selective” URAT1 inhibitor, an approach that’s seen as a way to improve upon existing therapies.
-

 Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Peter Marks, former top FDA vaccine official, joins Eli Lilly
Six months after his abrupt resignation as CBER director, Marks has been hired to run discovery and infectious disease work at the big Indianapolis drugmaker.
-
Deep Dive // Emerging biotech
Biotech startups are built on venture capital. Track funding rounds here.
Expedition Therapeutics, a startup aiming to in-license drugs from China, raised $165 million in the third nine-figure Series A round announced in the last three days.
Updated Oct. 9, 2025 -
Boehringer Ingelheim drug wins FDA OK in tough-to-treat lung disease
The clearance makes Jascayd the first new drug for idiopathic pulmonary fibrosis in over a decade, but some analysts see it as a “modest” advance in care.
-
Lexeo says FDA open to speedier approval of rare disease gene therapy
The agency will consider a submission that includes pooled data from ongoing studies, a decision analysts viewed as a notable, additional sign of regulatory flexibility for gene therapies.
-
IPO window
MapLight uses workaround to tee up IPO during government shutdown
The company set terms for an offering with the help of a little-used section of the Securities Act that would enable its IPO to price automatically in 20 days.
-


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration
 Tracker
Tracker5 FDA decisions to watch in the fourth quarter of 2025
Against the backdrop of a government shutdown, the agency has a series of critical decisions ahead, among them verdicts on new therapies from Novo Nordisk and Biohaven.
Updated Sept. 30, 2025 -
Obesity drugs
Skye shares crash as obesity drug falls short in key study
The findings are the latest setback for weight loss medicines that target a specific cannabinoid receptor and are meant to boost the effects of incretin therapies like Wegovy.
-
Trump administration
As shutdown begins, FDA to stop accepting new drug submissions
The funding lapse triggered by the U.S. government shutdown is the latest test for an agency that’s already dealt with significant layoffs and leadership upheaval this year.
-
AstraZeneca, Daiichi drug extends survival in hard-to-treat breast cancer
The results could pave the way for Datroway to supplant chemotherapy in certain triple-negative tumors, adding to existing clearances in other breast and lung malignancies.
-
China competition
Drugs from China are reshaping biotech. Track the licensing deals here.
Zenas’ deal is the second involving InnoCare and a U.S. or Europe-based drugmaker this year, following a pact with Prolium Bioscience in January.
Updated Oct. 8, 2025 -
News roundup
Gilead delays arrival of Biktarvy copycats; CDC updates vaccine schedule
A settlement could extend the exclusivity of the world’s best-selling HIV medicine to 2036. Elsewhere, an AstraZeneca drug acquisition paid more dividends and a Vertex veteran became Stoke’s full-time CEO.
-
Gene editing
Chiesi buys into Arbor gene editing drug for rare kidney disease
The Italian drugmaker will pay as much as $115 million in upfront and near-term payments in a deal that gives it rights to a treatment in early-stage testing for primary hyperoxaluria type 1.
-
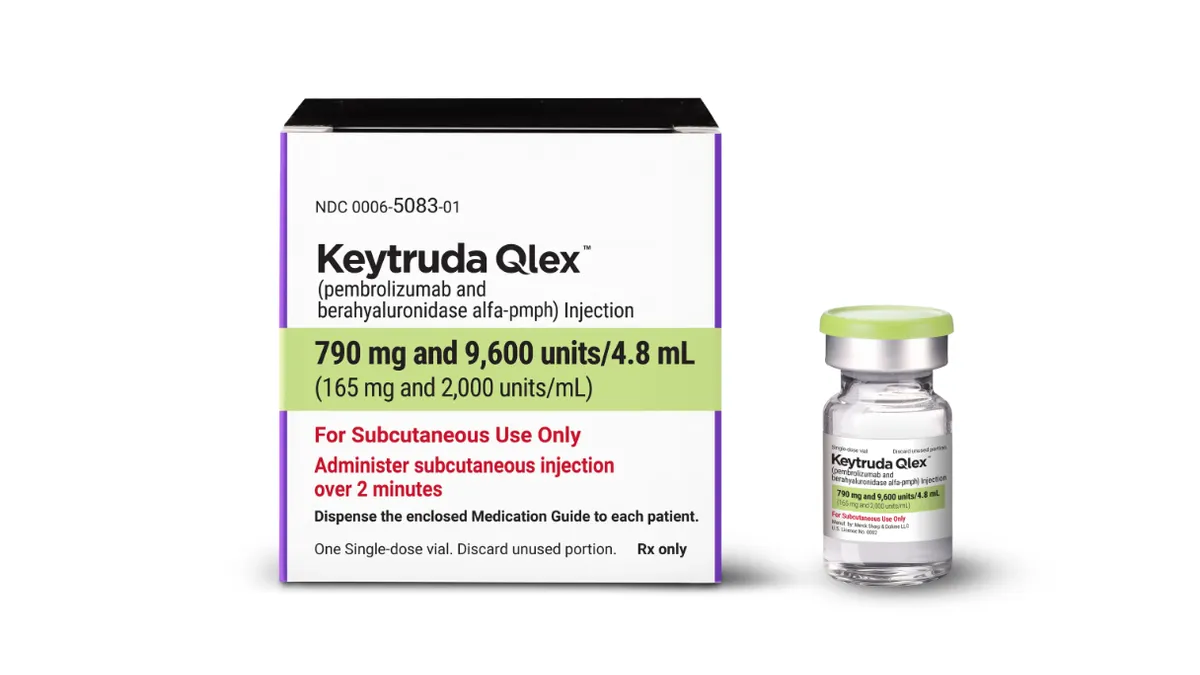
Half of Merck’s sales are in jeopardy. Can Keytruda’s sequel save the day?
Wall Street analysts expect the subcutaneous version of Keytruda, which just launched last week, to help soften the blow when the original loses patent protection later this decade.
-
News roundup
FDA clears generic abortion pill; CMS punts ‘combo drug’ guidance
The decision to clear another mifepristone generic sparked political backlash. Elsewhere, investors will pump up to $175 million into Ovid after the company reported early, but encouraging, data for a potential epilepsy drug.
-
Amgen claims ‘landmark’ study result that could widen heart drug’s use
In a first-of-its-kind finding, Amgen’s PCSK9 drug protected heart health when tested as a “primary prevention” therapy, which could pave the way for access to a much broader population.
-
Tracker
10 clinical trials to watch the rest of 2025
Expected readouts in obesity, lung cancer and AATD headline a series of study results that could give the biotechnology sector a boost.
Updated June 30, 2025 -
Deep Dive
AI could transform healthcare. Can safety-net providers keep up?
Implementing artificial intelligence requires significant human labor and technical expertise, threatening to create a digital divide between highly resourced health systems and safety-net providers.
-
Emerging biotech
Cartography secures $67M in pursuit of ‘differentiated’ cancer drugs
The company is working on T cell engagers and other multifunctional antibodies, led by a prospect it believes might be helpful treating the vast majority of colorectal cancer cases.
-
Takeda, in reversal, abandons cell therapy research
The Japanese pharmaceutical company, which had made cell therapy a priority a few years ago, aims to partner its work while prioritizing investments elsewhere.