Biotech: Page 73
-
Bluebird, after delays, gets speedy FDA review for beta thalassemia gene therapy
The regulator will decide whether to approve Bluebird's treatment by next May, but its evaluation is beginning more than a year later than the biotech hoped.
By Ned Pagliarulo • Nov. 22, 2021 -
Gilead pays up to retain rights to Arcus cancer drugs
The biotech will pay $725 million to opt into rights on four experimental medicines developed by Arcus, including ones that could challenge Merck's Keytruda in lung cancer.
By Jonathan Gardner • Nov. 18, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
A startup emerges with $125M and plans to edit the epigenome
Chroma Medicine's launch is the latest step in a decadeslong quest by drugmakers to capitalize on research into epigenetics, a way of controlling gene expression without altering DNA.
By Ben Fidler • Nov. 17, 2021 -
Roche cuts ties with Atea after COVID-19 pill's trial failure
The Boston biotech, shares of which soared last year on hopes for the antiviral drug, said it has plenty of money to advance development on its own.
By Kristin Jensen • Nov. 17, 2021 -
European drugs regulator signals rejection likely for Biogen's Aduhelm
A European Medicines Agency panel tasked with reviewing drugs voted against the biotech's controversial Alzheimer's medicine, signaling an approval is unlikely next month.
By Ned Pagliarulo , Jonathan Gardner • Nov. 17, 2021 -
Al Sandrock, Biogen's top scientist and Aduhelm defender, to retire in surprise exit
Sandrock championed the controversial Alzheimer's drug, and was integral to the development of several other of Biogen's most important medicines during his 24 years at the biotech.
By Jacob Bell • Nov. 16, 2021 -
Sponsored by IQVIA
Establishing an integrated evidence plan for medical affairs and beyond
Over the life of a biopharmaceutical product, there are many situations when data from randomized controlled trials (RCTs) may not be sufficient to address stakeholder questions of value, safety and effectiveness.
Nov. 15, 2021 -
Cortexyme plans path forward for Alzheimer's drug that failed study
Detailed trial results presented last week are "interesting," one Alzheimer's expert said, but there are still "many challenges and uncertainties" with Cortexyme's unorthodox approach to treating the disease.
By Ned Pagliarulo • Nov. 12, 2021 -
Moderna, escalating dispute with NIH, claims government had no role in key vaccine patent
The biotech claimed its NIH partners were involved only after a key discovery was made "by Moderna scientists using Moderna technology." The dispute could end up in court.
By Jonathan Gardner , Ben Fidler • Nov. 11, 2021 -

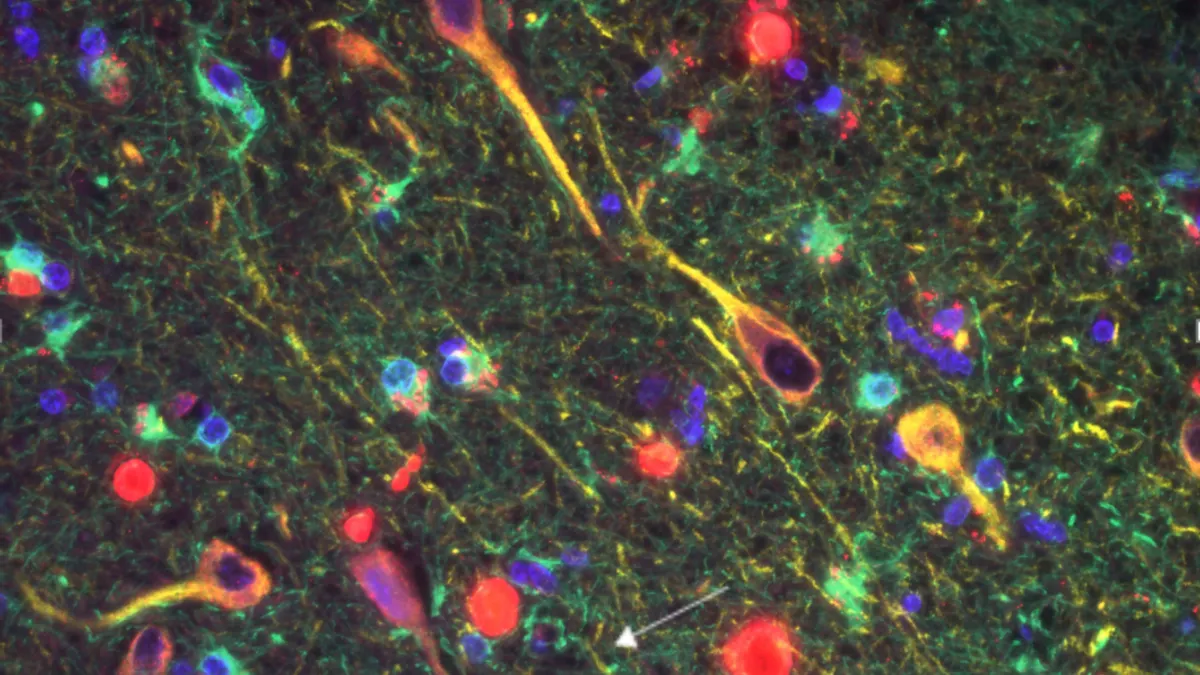
 National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
Detailed Roche study results muddy another Alzheimer's hypothesis
Data presented at an Alzheimer's meeting raised more questions about the seeming benefit of Roche and AC Immune's drug semorinemab, which they claim is the first of its kind to slow deterioration of memory.
By Jonathan Gardner • Nov. 11, 2021 -
FibroGen cuts jobs as it weighs anemia drug's future in US
The company, which had its treatment rejected by the FDA in August, eliminated 30 jobs and 70 open positions. An upcoming meeting with the agency looms as a key event.
By Kristin Jensen • Nov. 10, 2021 -
Pfizer buys into Biohaven's fast-selling migraine medicine
The pharmaceutical giant will pay Biohaven $500 million for ex-U.S. rights to rimegepant — a sizable licensing deal, though not the buyout that some of the biotech's investors appeared to be hoping for.
By Ned Pagliarulo • Nov. 9, 2021 -
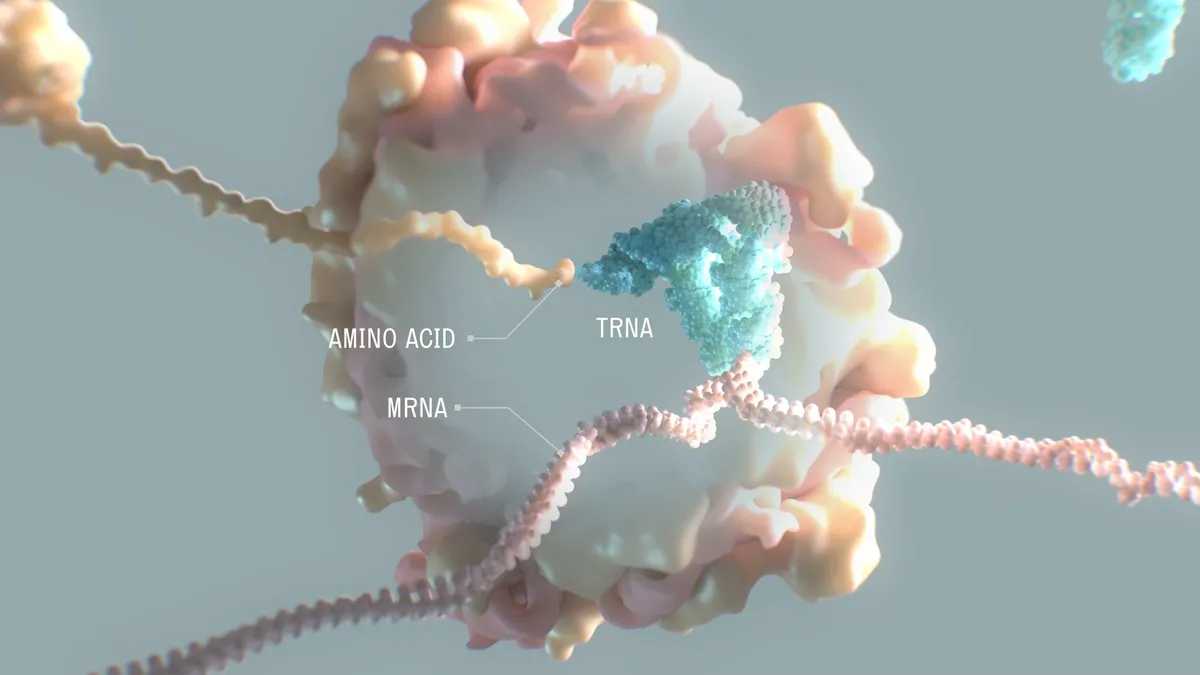
Moderna founder unveils new drug company focused on a different kind of RNA
Encouraged by Moderna's success, Flagship Pioneering has been busy creating startups like Alltrna, which launched Tuesday. The biotech is researching how to make medicines from transfer RNA molecules.
By Jacob Bell • Nov. 9, 2021 -

 Retrieved from National Cancer Institute on September 27, 2019
Retrieved from National Cancer Institute on September 27, 2019
Mirati gives first look at KRAS drug combination in lung cancer
Study results for Mirati's drug combined with Keytruda have been much anticipated, as the San Diego biotech aims to challenge Amgen and its rival KRAS-blocking drug Lumakras.
By Ned Pagliarulo • Updated Nov. 8, 2021 -
Eye-focused gene therapy startup gets $60M cash infusion from Sanofi
Shortly after postponing a $135 million IPO, Gyroscope has found another way to back a treatment for a disease that's become a top target of multiple drugmakers.
By Jonathan Gardner • Nov. 8, 2021 -
Beam gets green light to begin first clinical test of base editing
Alongside the milestone, the gene editing biotech also announced John Maraganore, who will soon step down as Alnylam's CEO, will join its board.
By Ned Pagliarulo • Nov. 8, 2021 -
Cystic fibrosis group partners with Moderna founder to find better, potentially curative treatments
With an initial investment of $20 million, the collaboration will use technologies from various Flagship-backed companies to discover and develop new therapies for the disease.
By Jacob Bell • Nov. 3, 2021 -
Moderna, teaming with a startup, wades further into gene editing
The biotech has identified gene editing as a natural fit for the technology it uses to deliver its vaccines and drugs. A deal with Metagenomi, a startup backed by Bayer, will further those ambitions.
By Ben Fidler • Nov. 2, 2021 -
Amylyx asks FDA to approve ALS drug, while also preparing late-stage study
The biotech has officially filed for review of its closely watched ALS treatment, an outcome that seemed unlikely just a few months ago.
By Jacob Bell • Updated Nov. 2, 2021 -
Moderna faces delay in plans to bring coronavirus vaccine to US teenagers, young kids
The FDA wants to better understand the risk of vaccine-associated myocarditis in 12- to 17-year-olds based on real-world use overseas, which will set back Moderna’s authorization plans in the U.S.
By Ben Fidler • Nov. 1, 2021 -
Despite strong quarter, some wonder how long Gilead can rely on coronavirus drug
While other drugs in Gilead’s arsenal fell short, third quarter revenue from Veklury tripled what analysts had expected, reaching nearly $2 billion.
By Jacob Bell • Oct. 29, 2021 -
John Maraganore, pioneering RNAi executive, to step down as Alnylam CEO
Alnylam shares fell 15% on the news, which surprised Wall Street analysts and ends Maraganore's 19-year run overseeing the company's journey from startup to a biotech worth nearly $20 billion.
By Ben Fidler • Oct. 28, 2021 -
Cortexyme drug fails in Alzheimer's trial, but company sees validation of unorthodox approach
Cortexyme shares lost more than three-fourths of their value after the the biotech's therapy, which is designed to work differently than drugs like Aduhelm, missed both primary goals in a late-stage study.
By Ned Pagliarulo • Oct. 26, 2021 -
Moderna vaccine safe and spurs immune response in kids, company says
The trial results should allow the company to ask the FDA for approval of its vaccine in younger children, just as Pfizer nears a crucial decision for its shot in 5- to 11-year olds.
By Jonathan Gardner • Oct. 25, 2021 -
Sponsored by IQVIA
Why natural language processing (NLP) is a critical part of your real world data strategy
The barrier to knowledge can be eliminated when pharmaceutical companies use natural language processing to analyze unstructured content.
By Hywel Evans, Director, IQVIA NLP Insights Hub • Oct. 25, 2021