Clinical Trials: Page 31
-
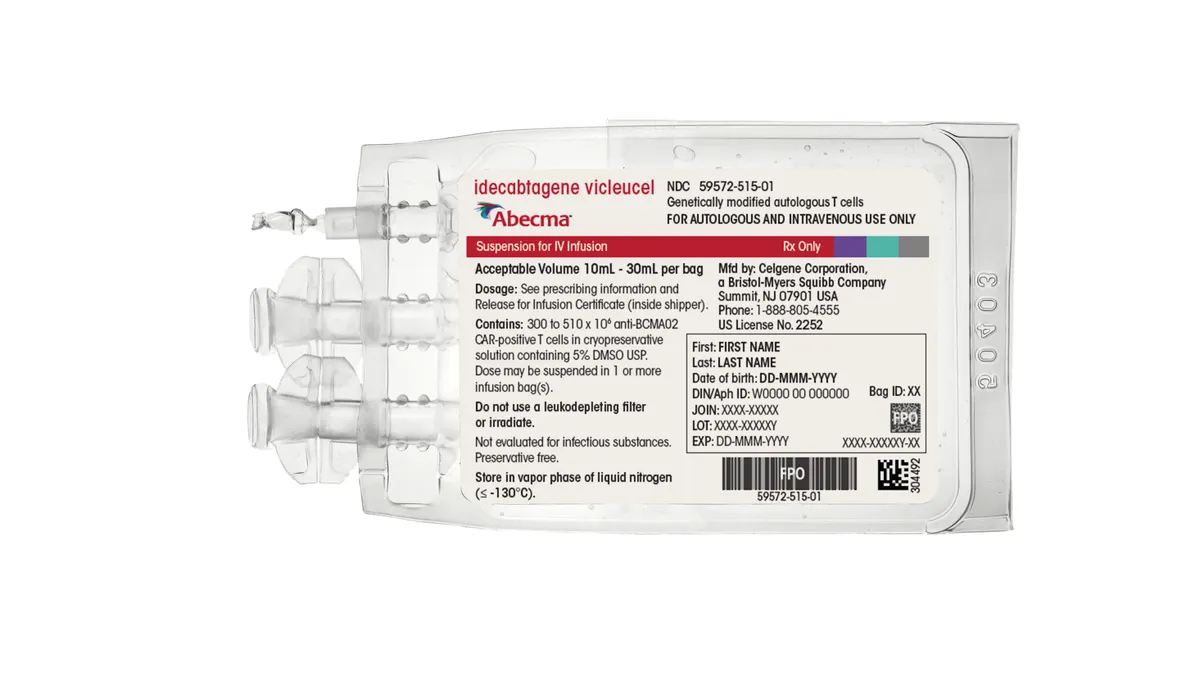
Bristol Myers claims success in study testing earlier CAR-T use in multiple myeloma
Abecma, which 2Seventy bio helps develop and sell, is currently approved for patients who have tried at least four other medicines for the blood cancer.
By Ned Pagliarulo • Aug. 10, 2022 -
Pfizer, Valneva take next step with Lyme disease shot, starting large trial
The experimental vaccine is the most advanced candidate for the tick-borne disease and, if successful, would be the first to reach the market since withdrawal of GSK’s Lymerix.
By Delilah Alvarado • Aug. 9, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineNeuroscience drug development
Enthusiasm is running higher among drugmakers and investors for neuroscience drug development, buoyed by recent approvals of new Alzheimer’s, ALS and depression medicines.
By BioPharma Dive staff -
Fresh off Enhertu success, AstraZeneca and Daiichi claim progress for next cancer drug
A treatment that AstraZeneca acquired for up to $6 billion in 2020 is showing early promise as a combination agent in lung cancer, where it’s being tested alongside Merck & Co.’s Keytruda.
By Ben Fidler • Aug. 9, 2022 -

 Retrieved from National Cancer Institute on September 27, 2019
Retrieved from National Cancer Institute on September 27, 2019
Amgen data on KRAS cancer drug disappoints, opening door for rival Mirati
Highly anticipated trial results for Lumakras combinations with immunotherapy fell short of expectations, showing significant liver toxicity that limited dosing.
By Ned Pagliarulo • Aug. 8, 2022 -
Lilly takes long view on Alzheimer’s drug as FDA starts expedited review
The pharma said Thursday the FDA had accepted its application for donanemab, starting a six-month evaluation for accelerated approval.
By Ned Pagliarulo • Aug. 5, 2022 -
Alnylam drug succeeds in key heart disease study, boosting company
The success of Alnylam's treatment in a trial known as APOLLO-B could pave the way for the biotech to reach consistent profitability, should the FDA sign off on an approval application.
By Ben Fidler • Aug. 3, 2022 -
Sponsored by Janssen Pharmaceuticals
The promise of oral therapies for cytokine inhibition
The science behind new oral therapies for cytokine inhibition and why they could be transformational for patients.
By David Lee, Global Therapeutic Area Head, Immunology • Aug. 1, 2022 -
Alnylam reveals longer wait for anticipated drug trial results
Important data from the APOLLO study of the biotech's drug Onpattro are due before the end of August, Alnylam said Thursday.
By Ned Pagliarulo • July 28, 2022 -
Pfizer, BioNTech to study 'bivalent' booster as FDA weighs approach
The small-scale study is part of the companies’ efforts to update their vaccine in time for a potential fall vaccination campaign to help boost waning immunity.
By Christopher Newman • July 27, 2022 -
BridgeBio advances drug for dwarfism after study data
The highest tested dose of BridgeBio’s drug helped the bones of children with achondroplasia grow faster, leading the company to expand study enrollment.
By Ned Pagliarulo • July 26, 2022 -
Seagen, Astellas claim positive results in study key to cancer drug's success
The mid-stage trial tested the companies’ drug Padcev with Merck’s Keytruda in first-line bladder cancer. The data are reportedly a factor in Merck’s talks with Seagen over a potential buyout.
By Ned Pagliarulo • July 26, 2022 -
Vertex to move non-opioid painkiller into late-stage tests after FDA agreement
Trial results released earlier this year showed potential for the drug, which has become one of Vertex’s top pipeline prospects.
By Delilah Alvarado • July 22, 2022 -
Atara shares sink after update on multiple sclerosis trial
Much-anticipated interim results from Atara’s Phase 2 study revealed little about the likelihood of the trial’s success, spurring a market sell-off.
By Ned Pagliarulo • July 13, 2022 -
With new data, Pliant drug shows promise in tough-to-treat lung disease
The drug’s potential impact on the lung health of idiopathic pulmonary fibrosis patients, many of whom take medicines from Roche and Boehringer Ingelheim, came as a “surprise” given the study’s small size, according to one analyst.
By Ben Fidler • July 11, 2022 -
New analysis encourages Intercept to refile NASH drug for approval
The company said the new examination is more “in line” with FDA guidance and still found that its drug, known as OCA, helped reduce liver scarring.
By Jacob Bell • July 7, 2022 -

 National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
FDA, under fire for Aduhelm approval, starts review of another Alzheimer's drug
The regulator could clear Biogen and Eisai’s lecanemab by early January. A decision before Phase 3 results could amplify the criticism the agency already faces, however.
By Jonathan Gardner • July 6, 2022 -
Deep Dive
10 clinical trials to watch in the second half of 2022
The biotech industry's downturn accelerated in the first half of the year. Important study readouts for Eisai, Gilead, Merck and Seagen, among others, could determine whether the slump endures.
By Ben Fidler , Ned Pagliarulo , Jacob Bell • July 5, 2022 -
Celldex, continuing comeback, adds to early promise for skin disease drug
New study results show the medicine appears as effective, or stronger, than standard care for chronic urticarias, a common condition that drugs from Regeneron and Novartis have struggled to treat.
By Ben Fidler • July 1, 2022 -
FDA halts tests of Sanofi drug, acquired in $3.7B buyout, due to side effects
Liver problems in trial volunteers led the agency to curtail studies of the drug, marking the second major setback related to Sanofi’s acquisition of Principia Biopharma.
By Jonathan Gardner • June 30, 2022 -
Jazz drug fails in late-stage study for multiple sclerosis
The drug, part of Jazz’s $7 billion acquisition of GW Pharmaceuticals, didn’t perform significantly better than placebo in treating the muscle spasticity that’s tied to the disease.
By Jacob Bell • Updated June 29, 2022 -
Sanofi, GSK say dual-acting vaccine prevents COVID-19 from omicron in large trial
A shot the partners have been developing reduced symptomatic infections associated with the variant by 72% compared to a placebo, positioning it as a potential option, if approved, to combat omicron.
By Jonathan Gardner • June 24, 2022 -
FDA suspends US testing of Sarepta Duchenne drug over safety concerns
The regulator stopped dosing in the U.S. for a drug that’s meant to be a more potent version of Sarepta's marketed medicine Exondys 51, after a patient experienced dangerously low magnesium levels.
By Ben Fidler • June 23, 2022 -
UniQure buoyed by early data for Huntington's gene therapy
After one year, researchers detected important protein changes in patients who received a low dose of the experimental treatment. Further testing and functional data are needed to assess its potential, however.
By Jonathan Gardner • June 23, 2022 -
Biogen, citing insurance challenges, shutters one of its Aduhelm studies
The company says a recent coverage decision by Medicare has forced it to end an observational trial of the Alzheimer's drug after enrolling just 29 participants.
By Jacob Bell • June 22, 2022 -
Mixed results for PTC Duchenne drug put spotlight on EU approval
A confirmatory study of PTC Therapeutics’ muscular dystrophy treatment Translarna missed its main goal, although the company highlighted the trial’s overall positive results.
By Ned Pagliarulo • June 21, 2022