Gene Therapy: Page 14
-
Lilly bets more than $600M on a gene therapy developer targeting hearing loss
Like with Prevail Therapeutics, Lilly’s first gene therapy acquisition, the pharma’s planned buyout of Akouos includes a contingent value right that could hike the deal’s overall cost.
By Jacob Bell • Oct. 18, 2022 -
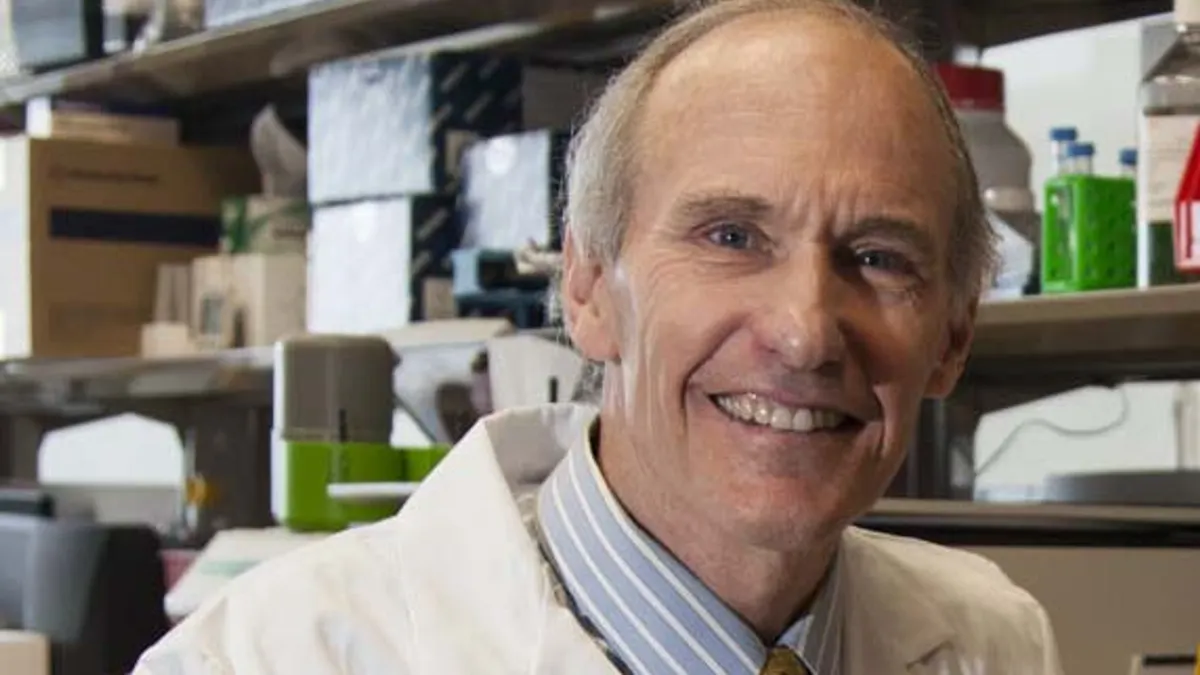
CAR-T pioneer Carl June on founding startups and cell therapy’s next act
The University of Pennsylvania immunologist and inventor of Kymriah spoke with BioPharma Dive about working with pharma, starting companies and the future of the cell therapy field.
By Ben Fidler • Oct. 18, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineGene Therapy
Rapid scientific advances have put the gene therapy field at the forefront of biomedical research. But, as recent setbacks have shown, researchers and drugmakers still face major challenges.
By BioPharma Dive staff -
State of Play
‘In vivo’ cell therapy: expanding beyond CAR-T
At least five startups have emerged with new ways to genetically modify immune cells within the body, an approach that, if successful, could widen the field of CAR-T treatment.
By Ben Fidler • Oct. 18, 2022 -

 Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
‘We have to find a way’: FDA seeks solutions to aid bespoke gene therapy
Gene therapies could help treat many ultra-rare diseases. But they may not get developed if drugmakers can’t build a sustainable business around them, CBER director Peter Marks said at a conference.
By Ned Pagliarulo • Oct. 13, 2022 -
BioMarin gets FDA decision date for hemophilia gene therapy, but questions on review remain
After issuing a surprise rejection of the therapy in 2020, the FDA has now agreed to again review BioMarin’s Roctavian therapy. An approval decision is expected by March 31.
By Kristin Jensen • Oct. 13, 2022 -
Ascidian starts up with $50M and a twist on RNA editing
The startup, run by Translate Bio cofounder Romesh Subramanian, believes its RNA “exon editing” approach could have long-lasting effects without the risks that come with editing DNA.
By Gwendolyn Wu • Oct. 12, 2022 -
Allogene starts first pivotal trials of an ‘off-the-shelf’ cell therapy for cancer
The biotech believes the two Phase 2 trials initiated Thursday can support approval applications for what could be the first allogeneic cancer cell therapy.
By Ben Fidler • Oct. 7, 2022 -
BioMarin resubmits its hemophilia gene therapy to the FDA
The resubmission has been long awaited after BioMarin’s original application was unexpectedly rejected by the FDA two years ago. The company expects a decision around the middle of next year.
By Delilah Alvarado • Sept. 30, 2022 -
Sarepta asks FDA to approve first gene therapy for Duchenne muscular dystrophy
The application comes a year earlier than previously had been expected, as company says drug reviewers are open to accelerated review based on data from early-stage trials.
By Jonathan Gardner • Sept. 29, 2022 -
Vertex given green light to seek US approval of CRISPR-based therapy
The company and its development partner, CRISPR Therapeutics, will begin submitting a rolling application in November. The blood disease treatment is the first of its kind to near an FDA review.
By Jacob Bell • Sept. 27, 2022 -
Pfizer, Sangamo set to resume gene therapy study after safety delay
Concerns over blood clotting risk had derailed testing of the hemophilia treatment last year, leading the companies to adjust their trial protocol.
By Delilah Alvarado • Sept. 23, 2022 -
Back-to-back gene therapy approvals give Bluebird shot at survival
The FDA’s clearances of Zynteglo and Skysona are a boost to Bluebird, and could help lift the research field after a series of setbacks. But selling the high-priced therapies will be a challenge.
By Ned Pagliarulo • Sept. 19, 2022 -
Bluebird wins FDA approval of gene therapy for rare brain disorder
The therapy, called Skysona and cleared to treat cerebral adrenoleukodystrophy, is the product of more than a decade of work by Bluebird. It will cost $3 million per patient.
By Ned Pagliarulo • Updated Sept. 17, 2022 -
BioMarin reports cancer case in hemophilia gene therapy trial
The development comes three weeks after Roctavian was approved in Europe and ahead of a planned regulatory submission in the U.S. Drug regulators have not ordered a trial hold, however.
By Jonathan Gardner • Sept. 13, 2022 -
Mitochondrial drugs, with a twist: Pretzel Therapeutics launches with $72.5M in funding
Scientists at Pretzel believe fixing mutated mitochondrial DNA with a mix of small molecule therapies and gene editing could be key to solving a number of hard-to-treat diseases.
By Gwendolyn Wu • Sept. 12, 2022 -
Beam-linked startup launches with an eye on the next generation of RNA drugs
Orbital co-founder and former Alnylam CEO John Maraganore said the startup aims to “overcome some of the shortcomings that have been there with the first generation of RNA companies.”
By Gwendolyn Wu • Sept. 7, 2022 -
Sangamo presses ahead with Fabry disease gene therapy
Preliminary results from a Phase 1 study show Sangamo’s treatment to be safe and suggest it is working as intended, leading the biotech to move into the trial’s next phase.
By Ned Pagliarulo • Sept. 1, 2022 -
Beam details reasons for FDA hold on base editing cancer therapy
The regulator has asked Beam for a range of technical data before it can start testing the blood cancer treatment in humans.
By Kristin Jensen • Aug. 31, 2022 -
With European approval secured, BioMarin puts roughly $1.5M price tag on hemophilia gene therapy
On a conference call, BioMarin executives revealed the anticipated price for Roctavian and the company's initial launch plans, which include pay-for-performance deals customized to different markets.
By Jacob Bell • Aug. 25, 2022 -
ElevateBio finds a new manufacturing home and long-term gene therapy partner in Pittsburgh
The biotech signed a 30-year deal with the University of Pittsburgh to establish a gene and cell therapy manufacturing hub that’s being built with a $100 million grant.
By Kristin Jensen • Aug. 25, 2022 -
Ovid turns to gene therapy startup to restock drug pipeline
The New York biotech will invest in and develop up to three drugs with Gensaic, an emerging startup aiming to use the viruses that infect bacteria to deliver genetic medicines.
By Ben Fidler • Aug. 24, 2022 -
With $2.8M gene therapy, Bluebird sets new bar for US drug pricing
Approved for severe beta thalassemia, Zynteglo will test insurers’ willingness to pay for expensive one-time treatments. Its market launch is likely to be watched carefully by other gene therapy developers.
By Ned Pagliarulo • Aug. 18, 2022 -
Bluebird gene therapy approved by FDA for rare blood disease
The regulator cleared the biotech’s medicine Zynteglo for transfusion-dependent beta thalassemia, giving patients a powerful new treatment option. But it will come at a cost of $2.8 million in the U.S.
By Ned Pagliarulo • Updated Aug. 17, 2022 -
Gene therapy safety
Novartis reports deaths of two patients treated with Zolgensma gene therapy
The deaths were due to acute liver injury, a known risk of Zolgensma and a concern for gene therapies like it.
By Ned Pagliarulo • Aug. 11, 2022 -
GentiBio partners with Bristol Myers as cell therapy for immune disease gains momentum
The deal, worth as much as $2 billion, is the latest sign of industry interest in treatments that harness regulatory T cells, an approach behind several recent biotech startups.
By Ben Fidler • Aug. 10, 2022