Clinical Trials: Page 47
-

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
Oxford coronavirus vaccine spurs immune responses, appears safe on first look
The vaccine, from the University of Oxford and AstraZeneca, is backed with more funding from governments and non-profit groups than any other in development.
By Ben Fidler • July 20, 2020 -
New data from MeiraGTx help bolster J&J's gene therapy bet
The results are the first from a slate of gene therapies J&J licensed from Meira last year, a deal that remains the pharma's largest investment in the field.
By Ben Fidler • July 17, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineOncology's research boom
More than one quarter of the medcines cleared by the FDA's main review office since 2015 have been cancer drugs, a tally that reflects the advent of cancer immunotherapy as well as continued progress in matching treatment to genetics.
By BioPharma Dive staff -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
J&J, on the cusp of starting coronavirus vaccine trials, sets high bar for success
On a second quarter earnings call, J&J's top scientist said he expects the company's vaccine to significantly surpass the effectiveness standard set out by the FDA.
By Ned Pagliarulo • July 16, 2020 -
Researchers publish long-awaited study data on Moderna coronavirus vaccine
The results support the biotech's earlier claim of positive results, but don't show whether the vaccine protects against infection. Still, "we're so hungry for a vaccine we're trying to read the tea leaves," said vaccine expert Paul Offit.
By Ned Pagliarulo • Updated July 14, 2020 -
Preventive studies for Eisai, Biogen Alzheimer's drug could add years to amyloid debate
Two late-stage trials will assess the companies' experimental drug BAN2401 in people without Alzheimer's symptoms but with biological signs of the disease.
By Jonathan Gardner • July 14, 2020 -
Sanofi turns to MD Anderson to help fuel cancer drug push
The French pharma is counting on a five-year alliance with the cancer center to speed its early oncology research, a renewed focus under CEO Paul Hudson.
By Ned Pagliarulo • July 14, 2020 -
Spark, chasing BioMarin, plans 2021 start for key hemophilia gene therapy study
The Roche subsidiary said it will begin Phase 3 dosing its hemophilia A gene therapy next year, potentially putting it well behind a competing treatment from BioMarin.
By Jonathan Gardner • July 13, 2020 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from https://www.flickr.com/photos/nihgov/49565662436/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from https://www.flickr.com/photos/nihgov/49565662436/in/album-72157713108522106/.
India clears a psoriasis drug for COVID-19, and a tiny US biotech's shares soar
An emergency approval for Biocon's drug Alzumab in India could lead its U.S. partner, Equillium, to start broader trials in coronavirus disease.
By Ben Fidler • July 13, 2020 -
FDA documents reveal doubts about GSK blood cancer drug
Eye-related side effects could derail the leading drug that homes in on a new multiple myeloma target, just as rival therapies progress toward FDA review.
By Jonathan Gardner • July 10, 2020 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from https://www.flickr.com/photos/nihgov/49565662436/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from https://www.flickr.com/photos/nihgov/49565662436/in/album-72157713108522106/.
In the UK, a coronavirus study shows how to get fast, powerful data during a pandemic
RECOVERY, a large trial of COVID-19 treatments in the U.K., produced much of what the world knows about which medicines work and which do not.
By Ned Pagliarulo • July 10, 2020 -
ALS drug development
In the hunt for ALS treatments, researchers find promise in silencing genes
Two studies published in the New England Journal of Medicine, including one that tested a Biogen drug, provide some evidence that a decades-long effort to develop genetic medicines for ALS is starting to pay off.
By Jacob Bell • July 8, 2020 -
Novavax gets $1.6B in US funding for coronavirus vaccine
The funding will secure 100 million doses of Novavax's experimental shot, which is set to begin late-stage testing this fall.
By Ned Pagliarulo • July 7, 2020 -
Regeneron advances COVID-19 antibody cocktail into late-stage trials
The launch of a Phase 3 prevention study accelerates work on antibody-based drugs, which could offer strained healthcare systems a "bridge" to a vaccine.
By Jonathan Gardner • July 6, 2020 -
Sponsored by PRA Health Sciences
Collaboration, not competition, will bring better pediatric medicines to market
Drug developers and stakeholders must find ways to collaborate, rather than compete, as the RACE Act prompts the need for more pediatric research.
July 6, 2020 -
Coronavirus vaccine from Pfizer, BioNTech shows early potential
Initial data from a small study of healthy volunteers show the companies' shot can elicit immune responses against the virus.
By Ned Pagliarulo • July 1, 2020 -
FDA insists it 'will not cut corners' in coronavirus vaccine approvals
New guidelines released by the agency detail what reviewers will require from drugmakers before approving or making available any coronavirus vaccine.
By Ned Pagliarulo • June 30, 2020 -
 National Institute of Allergy and Infectious Disease. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Disease. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
Without data, Inovio says study shows positive results for coronavirus vaccine
The biotech is among the first few companies to disclose preliminary trial results for a coronavirus shot. But Inovio shared few details, making its announcement difficult to assess.
By Jonathan Gardner • June 30, 2020 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49565158908/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49565158908/in/album-72157713108522106/.
Large UK study finds no benefit to HIV antiviral in COVID-19
The results are the third significant clinical finding from the RECOVERY study, which recently determined the steroid dexamethasone improved survival.
By Ned Pagliarulo • June 30, 2020 -
Third patient dies in halted study of Audentes gene therapy
The three patients, treated for a rare neuromuscular disease, were given a high dose of Audentes' gene therapy.
By Ben Fidler • Updated Aug. 21, 2020 -
WHO lays out ambitious plan to deliver 2 billion coronavirus vaccine doses
More than $18 billion will be needed for the WHO's plan, which would secure and allocate vaccine supplies for high-risk groups and lower-income countries.
By Ned Pagliarulo • June 26, 2020 -
Sponsored by PSI CRO
Clinical trials are only getting more complex, and wishful thinking has to go
If you look at the data trends over the last decade, there's no avoiding the truth: clinical trials are getting more complex every year.
By Natania Barron Global Director, Marketing • June 26, 2020 -
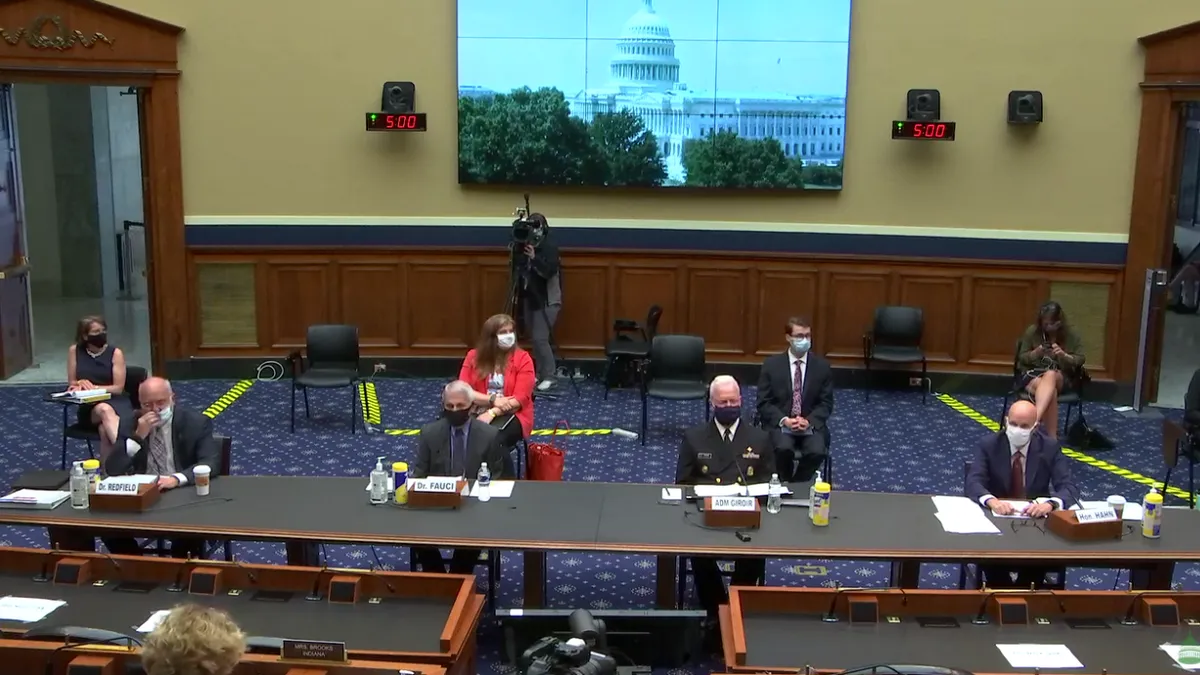
Fauci 'cautiously optimistic' about coronavirus vaccine by 2021
Anthony Fauci, the nation's top infectious disease official, told lawmakers the next few weeks will be critical as COVID-19 cases rise in many states.
By Rebecca Pifer • June 24, 2020 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
NIH ends key COVID-19 studies of hydroxychloroquine
The agency stopped one trial because the malaria drug didn't appear to be helping patients, and halted the second because few people were enrolling.
By Jonathan Gardner • June 22, 2020 -
Merck gives 1st look at Phase 3 results for its Prevnar competitor
Results from two late-stage studies of V114, Merck's experimental pneumococcal conjugate vaccine, could help support a submission for approval later this year.
By Ned Pagliarulo • June 22, 2020 -
UniQure gets out the gate first in race for Huntington's gene therapy
The biotech has dosed patients in the first trial of a gene-targeted treatment for the degenerative disease, but competitor Voyager could be chasing soon.
By Jonathan Gardner • June 19, 2020