FDA: Page 26
-
Deep Dive // New Alzheimer's drugs
In historic move, FDA approves a closely watched and controversial Alzheimer's drug
The decision cleared the way for what many predicted would become a lucrative treatment. But a high price and controversy over whether Biogen's Aduhelm benefits patients weighed heavily on the drug's launch.
By Jacob Bell • Updated June 7, 2021 -
Former FDA official Abernethy joins Google health spinoff
The agency's former acting CIO pushed development of real world evidence as well as improved data sharing. At Verily, she'll head up the unit's clinical research business.
By Jonathan Gardner • June 3, 2021 -
Alkermes prepares for uncertain launch as FDA finally clears schizophrenia drug
Lybalvi is meant to be as effective as marketed antipsychotics but without the weight gain patients typically experience. Alkermes will introduce the drug into a competitive market, however.
By Jacob Bell • June 1, 2021 -

 Retrieved from National Cancer Institute on September 27, 2019
Retrieved from National Cancer Institute on September 27, 2019
In first, FDA approves KRAS-blocking cancer drug from Amgen
For decades, scientists have tried unsuccessfully to target the KRAS gene, which is often mutated in lung, colon and pancreatic cancers. Lumakras is the first drug proven effective.
By Ned Pagliarulo • Updated May 29, 2021 -
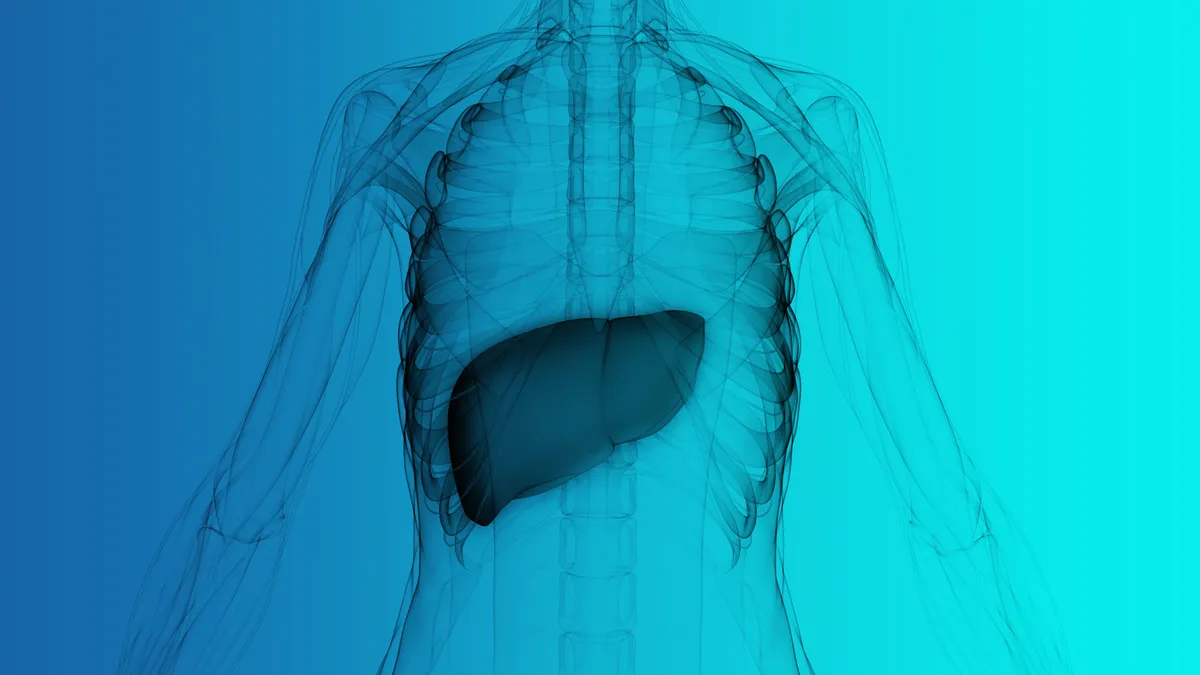
FDA restricts use of Intercept drug due to liver injury risk
An investigation linked the drug to severe injuries in about two dozen patients, leading the agency to add a new contraindication to the label.
By Kristin Jensen • May 27, 2021 -
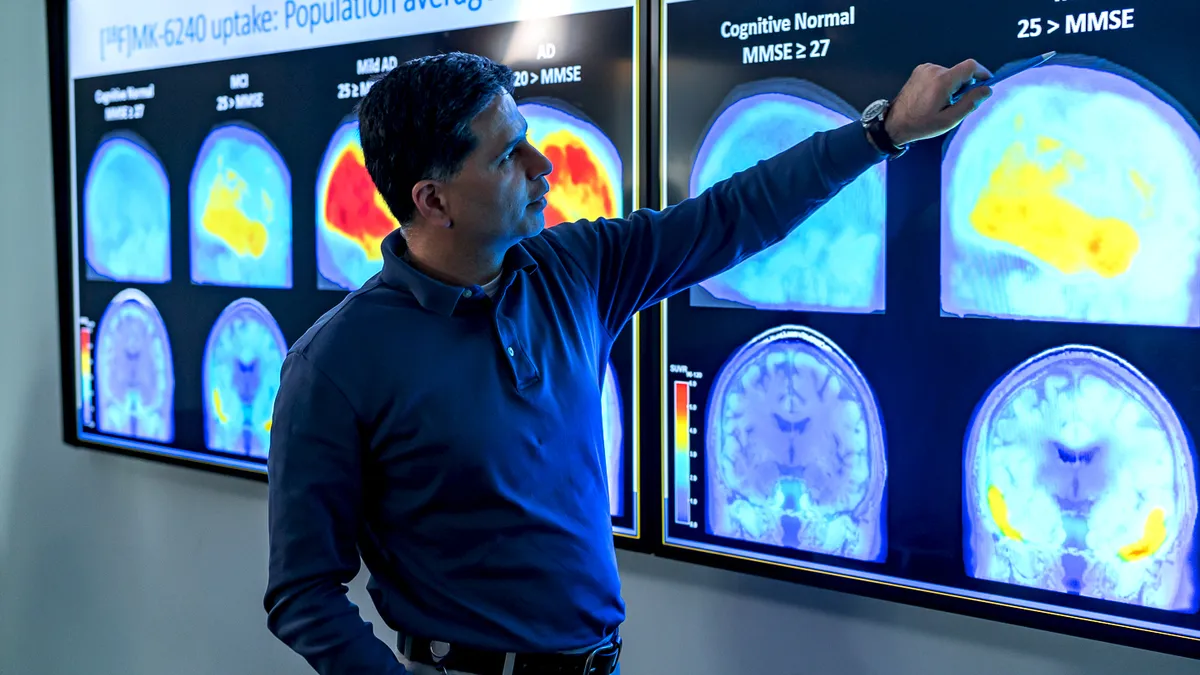
'A hugely consequential decision:' How Biogen's Alzheimer's drug came to face the FDA
The FDA's decision to approve aducanumb could have far-reaching consequences for patients, Biogen and Alzheimer's research. Here's how the drug's review came about.
By Ned Pagliarulo • May 27, 2021 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
Vir, GSK win US nod for another COVID-19 antibody drug as rival falters
Vir's sotrovimab arrives months after similar treatments from Eli Lilly and Regeneron. But the therapy could still play a role due to its apparent potency against variants that have challenged Lilly's drugs.
By Ben Fidler • May 27, 2021 -
Coronavirus variants threaten to undermine another Lilly COVID-19 drug
One month after Lilly's first coronavirus antibody was pulled from market, the U.S. government stopped distributing its other treatment in two states.
By Ben Fidler • May 24, 2021 -
FDA seeking more consistency from cell, gene therapy developers, top official says
Several drugmakers have recently faced development delays after the FDA's asked for more information on how they measure the potency of their products.
By Ned Pagliarulo • May 19, 2021 -
Merck, with new breast cancer data, aims to rebound from FDA rejection
Study results could address concerns raised by FDA advisers who recently voted against approval of Keytruda in patients with early-stage breast cancer.
By Ben Fidler • May 13, 2021 -
Heron, on third attempt, secures FDA approval for non-opioid painkiller
The San Diego-based biotech's drug, which will now be sold as Zynrelef, had perviously been rejected by the agency in 2019 and 2020.
By Kristin Jensen • May 13, 2021 -
FDA unexpectedly grounds a gene therapy for a rare heart disease
Rocket Pharma's Danon disease treatment — key to the company's quiet rise over the past year — is the latest gene therapy to be put on hold by the agency. Executives predict only a short delay, however.
By Ben Fidler • May 11, 2021 -
FDA authorizes Pfizer's coronavirus vaccine for younger teens
The emergency clearance greatly expands the pool of people who can be vaccinated in the U.S. just as some states begin to report waning demand.
By Ned Pagliarulo • May 10, 2021 -
Pfizer, BioNTech are first to seek full FDA approval of a coronavirus vaccine
The milestone filing could pave the way for the shot's use beyond the pandemic and give employers the legal heft to require vaccination, a key step toward herd immunity in the U.S.
By Jonathan Gardner • May 7, 2021 -
FDA faces tough choice after panel backs speedy cancer drug approvals
The April meeting followed several immunotherapy withdrawals and led to multiple others, part of a push by the agency to review "dangling accelerated approvals."
By Ben Fidler , Ned Pagliarulo • April 30, 2021 -
Denying problems, AstraZeneca says US coronavirus vaccine filing due within weeks
Five weeks after AstraZeneca reported positive trial results, the company has still not applied to the FDA for authorization, saying the size of the dataset has slowed its submission.
By Jonathan Gardner • April 30, 2021 -
FDA gives first citation to biotech for failure to report clinical trial details
The agency threatened to fine Acceleron for not posting study results to clinicaltrials.gov. Whether the action is a sign of a larger crackdown is unclear.
By Kristin Jensen • April 29, 2021 -
Lilly, citing FDA feedback, won't seek speedy approval of Alzheimer's drug
The drugmaker confirmed it won't try for accelerated approval of a closely watched Alzheimer's medicine based on a single Phase 2 trial. But it's planning a lengthy new study in presymptomatic patients.
By Jonathan Gardner • April 27, 2021 -
FDA lifts hold on UniQure gene therapy study after review of cancer case
An investigation by UniQure determined the company's hemophilia gene therapy was "highly unlikely" to have caused a study volunteer's liver cancer, clearing the way for the FDA's green light.
By Jonathan Gardner • April 26, 2021 -
FDA, CDC support resuming use of J&J vaccine after advisory panel vote
While health officials have documented more cases of a rare blood clotting syndrome associated with J&J's vaccine, a CDC committee supported use of the shot with an added warning.
By Ned Pagliarulo , Ben Fidler • Updated April 23, 2021 -
GSK immunotherapy wins FDA approval, joining crowded cancer drug class
Jemperli is the seventh drug cleared by the FDA that blocks either PD-1 or PD-L1 proteins, joining Merck's Keytruda, Bristol Myers' Opdivo and others.
By Ned Pagliarulo • April 23, 2021 -
A look ahead at the FDA meeting that could decide the future of 6 cancer drug approvals
Accelerated approvals for immunotherapies from Merck, Bristol Myers Squibb and Roche might soon be withdrawn. Here's a detailed look at why.
By Ben Fidler , Ned Pagliarulo , Jonathan Gardner • April 22, 2021 -
Lilly asks FDA to revoke clearance of first COVID-19 antibody drug
The request comes less than six months after the FDA authorized Lilly's bamlanivimab, reflecting the spread of virus variants that can elude the drug.
By Ben Fidler • April 16, 2021 -
The US paused use of J&J's vaccine. What happens next?
A call by regulators to stop J&J vaccinations won't dramatically disrupt supply in the U.S. But changes in labeling are possible, as is a renewed debate over vaccine hesitancy.
By Ned Pagliarulo , Ben Fidler , Jonathan Gardner • April 13, 2021 -
US recommends pause in J&J vaccinations due to concerns over rare blood clots
Regulators identified rare and serious blood clots in six women vaccinated with J&J's shot. The reaction resembles a side effect linked to AstraZeneca's vaccine.
By Ben Fidler , Ned Pagliarulo • Updated April 13, 2021