Marketing: Page 3
-
Vaccines
Experts ousted from CDC panel warn of damage to US vaccine policy
The 17 advisers fired in June by Robert F. Kennedy Jr. urged alternatives to ACIP be organized to limit further degradation in vaccine guidelines.
By Delilah Alvarado • July 31, 2025 -
Alnylam reaches new highs on strong sales of closely watched rare disease drug
Despite battling drugs from BridgeBio and Pfizer, Amvuttra beat Wall Street expectations with results Alnylam’s CEO claimed were “not a flash in the pan.”
By Ben Fidler • July 31, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Brian Tucker / BioPharma Dive/BioPharma Dive
Brian Tucker / BioPharma Dive/BioPharma Dive Trendline
TrendlineCommercialization
New drugs for obesity are becoming blockbusters, while Trump administration pressure is reshaping pharma marketing strategies ahead of looming patent cliffs.
By BioPharma Dive staff -
J&J tests direct CAR-T marketing with first Carvykti ad
The new direct-to-consumer ad signals J&J’s confidence in its capacity to meet demand for the cell therapy after earlier manufacturing struggles.
By Ned Pagliarulo • Updated July 30, 2025 -
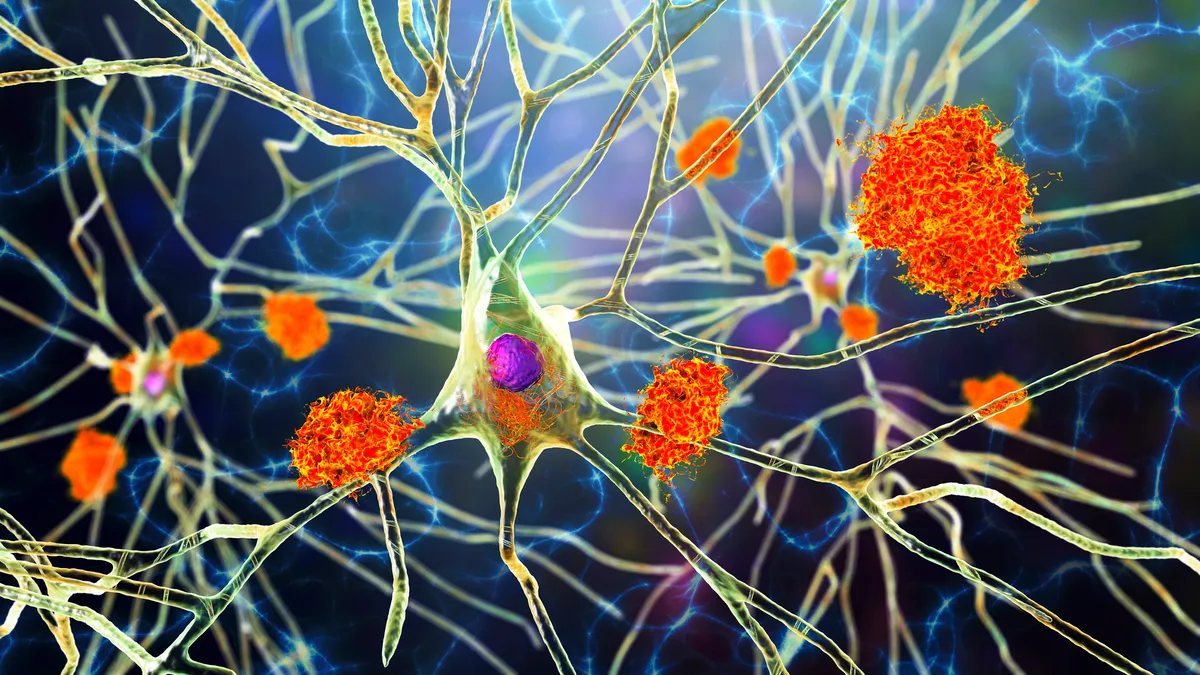
Brain drug revival
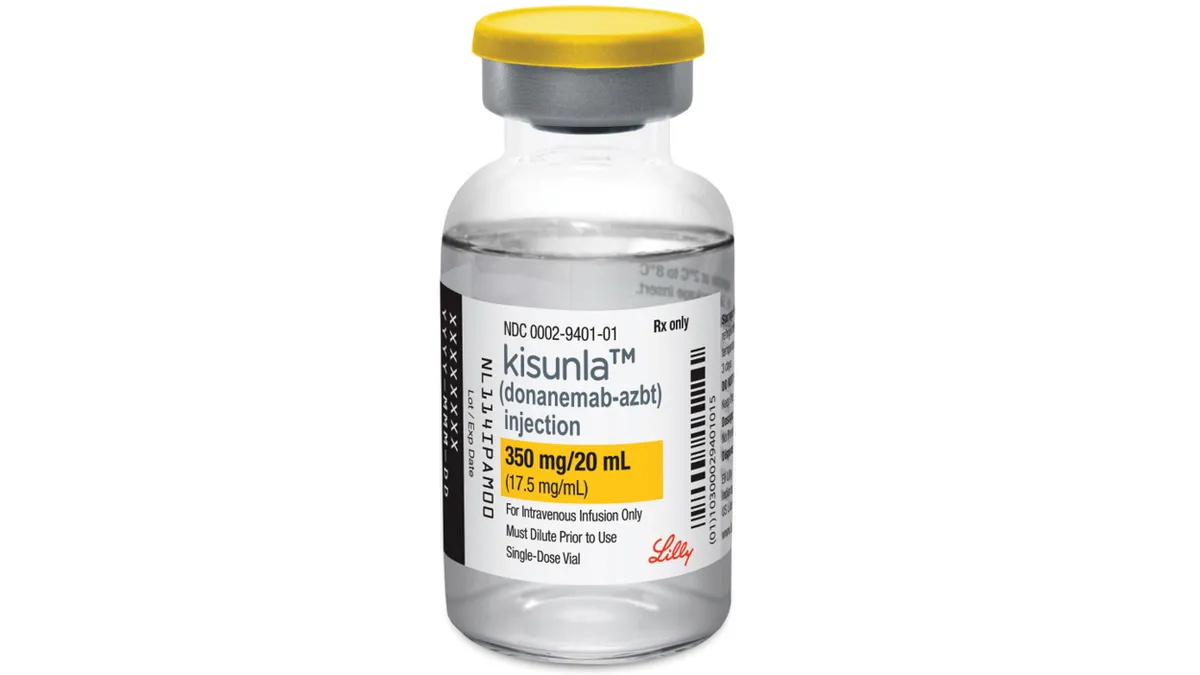
In reversal, European regulators take positive view on Lilly Alzheimer’s drug
Lilly’s appeal of an earlier, negative recommendation from a key EMA committee has worked out in its favor, and puts the drug, Kisunla, one step closer to European market entry.
By Jacob Bell • July 25, 2025 -
Vaccines
RFK Jr. adopts CDC panel recommendation to remove thimerosal from flu shots
The action will remove from use the very few flu shots that contain the contested preservative, elevating unproven fears it poses health risks.
By Delilah Alvarado • July 23, 2025 -
Bristol Myers, Pfizer to offer Eliquis at a discount for some patients
The program is the latest example of pharmaceutical companies bypassing traditional drug distribution channels for people who aren’t insured or are willing to pay cash for their medications.
By Ben Fidler • July 17, 2025 -
Soleno sales of new Prader-Willi drug rise faster than expected
Preliminary quarterly sales figures for Vykat surpassed Wall Street estimates, but shares fell on concerns the company may not be courting a buyer.
By Ben Fidler • July 10, 2025 -
Lilly gets FDA OK of modified dosing for Alzheimer’s drug
A slower ramp-up of Kisunla dosing lowers the rate of dangerous brain swelling, a risk that has made doctors reluctant to prescribe Lilly’s amyloid-busting drug.
By Jonathan Gardner • July 9, 2025 -
Trump administration
Medical groups, pregnant doctor sue RFK Jr. over vaccine changes
The complaint argues Kennedy’s actions to remove the COVID vaccine from the CDC's immunization schedule for pregnant people and healthy children were unlawful.
By Delilah Alvarado • Updated July 8, 2025 -
Q&A // Brain drug revival
Acadia CEO sets sights on ‘much more assertive’ deals to invigorate pipeline
Industry veteran Catherine Owen Adams expects external innovation will be key to getting the brain-focused drug developer to “the next level.”
By Jacob Bell • July 2, 2025 -
FDA takes major step to ease access to CAR-T therapy
The agency removed some onerous requirements for the complex cancer drugs and reduced restrictions on patients’ post-treatment movement.
By Ned Pagliarulo • June 27, 2025 -
Supreme Court upholds ACA preventive services mandate
The high court preserved a key part of the Affordable Care Act requiring private insurers cover a range of preventive healthcare services without cost sharing.
By Emily Olsen • June 27, 2025 -
Congress should reconsider breaking up PBMs, experts say
Many proposals to reform the PBM industry miss the forest for the trees, experts said this week. Instead, Congress should go after the oligopoly enjoyed by the “Big Three” companies.
By Rebecca Pifer Parduhn • June 27, 2025 -
Vaccines
RFK Jr.-appointed panel recommends flu shots be free of contested preservative
Thimerosal, targeted by activists who claim it’s linked to autism, shouldn't be used in vaccines given in the U.S. this coming flu season, the CDC panel said.
By Jonathan Gardner • Updated June 27, 2025 -
Recast CDC panel recommends new RSV antibody for infants
By a 5-2 vote, the Advisory Committee on Immunization Practices endorsed Merck’s Enflonsia for babies born during or entering their first RSV season.
By Ned Pagliarulo • June 26, 2025 -
Obesity drugs
Novo abruptly ends obesity drug deal with Hims
The Wegovy maker says the telehealth company didn't stop selling compounded versions of the blockbuster drug after Novo resolved its shortage.
By Jonathan Gardner • Updated June 23, 2025 -
Vaccines
RFK Jr. reveals picks for influential vaccine panel
Days after firing all 17 members of the CDC's Advisory Committee for Immunization Practices, the HHS Secretary named a small new committee that includes multiple vaccine critics.
By Kristin Jensen • June 12, 2025 -
FDA clears Nuvation lung cancer drug, setting up battle with Bristol Myers and Roche
The drug, Ibtrozi, will now vie for market share in an indication where multiple large pharmaceutical companies have already struggled to grow sales.
By Ben Fidler • June 11, 2025 -
ASCO25
AI tool could help doctors ID breast cancers vulnerable to Enhertu
Tumors with low- and ultra-low levels of a protein called HER2 are treatable with Enhertu, but harder to identify. New research shows AI can improve diagnosis.
By Ned Pagliarulo • May 22, 2025 -
Judge shuts down drugmakers’ 340B rebate plans, for now
A district court ruled HHS has the power to review drugmakers’ plans to pay hospitals rebates on 340B medications. But it left the door open to the controversial reforms.
By Rebecca Pifer Parduhn • May 19, 2025 -
FDA OKs first blood test to aid Alzheimer’s diagnosis
The FDA cleared the test for early detection of amyloid plaques associated with Alzheimer’s in people aged 55 years and older with signs and symptoms of the disease.
By Nick Paul Taylor • May 19, 2025 -
Andrew Witty steps down as UnitedHealth CEO
Witty’s departure was unexpected, but follows a downturn in the healthcare company’s financial outlook. He will be replaced by Stephen Hemsley, chair of UnitedHealth’s board.
By Rebecca Pifer Parduhn • May 13, 2025 -
Medicaid cuts could have ‘drastic impact’ on providers
Some providers may need to reduce services, lay off staff or close their doors if Congress enacts major cuts to the safety-net insurance program, experts say.
By Emily Olsen • May 9, 2025 -
Amgen’s Stelara biosimilar gets off to fast start
Sales of the company’s copycat to J&J’s blockbuster reached $150 million in the first quarter, adding to a surge in revenue for Amgen's biosimilar business.
By Jonathan Gardner • May 2, 2025 -
CVS strikes Wegovy deal with Novo Nordisk
The deal could expand access to its in-demand obesity drug at the expense of Eli Lilly, whose shares fell by double digits on Thursday.
By Rebecca Pifer Parduhn • May 1, 2025