Biotech: Page 78
-
Sponsored by Millipore-Sigma®
Incorporating automation and digitalization into the industrial microbiology workflow
A closer look at the impact of automated and digitalized technologies on the industrial microbiology workflow.
By Brad Grobler, Director of Strategic Initiatives, Biomonitoring Solutions at MilliporeSigma • June 21, 2021 -
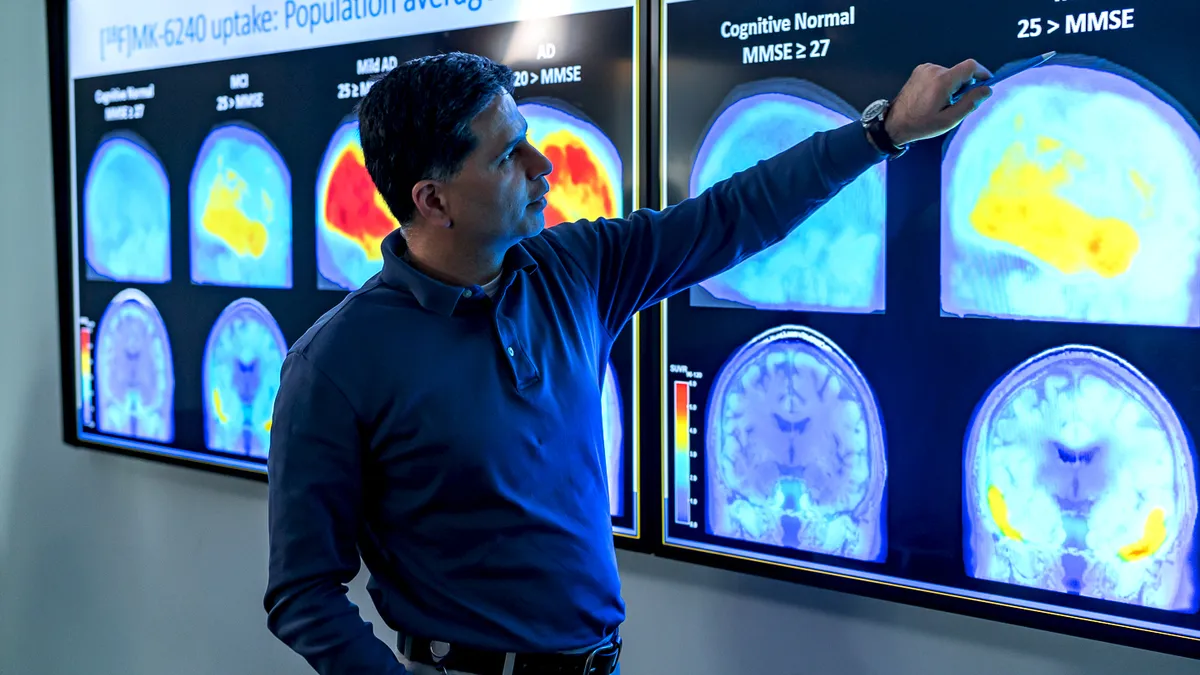
Trial failure dashes Biogen hopes for next Alzheimer's drug
Days after Aduhelm's approval, Biogen said an experimental drug that works in a different way didn't help patients, leading the company to stop research.
By Jonathan Gardner • June 17, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
FDA approves Blueprint cancer drug for use in rare blood disease
The agency's decision makes available a new treatment option for patients with advanced systemic mastocytosis, boosting the biotech in the process.
By Kristin Jensen • June 17, 2021 -
Gene editing biotech Verve to raise $267M in large IPO
Capitalizing on investor interest in gene editing, the high-profile startup has secured one of the richest biotech initial public offerings priced this year.
By Ned Pagliarulo • June 17, 2021 -
CureVac vaccine disappoints in key study amid 'unprecedented' spread of variants
The German biotech’s shot appeared less effective than other mRNA vaccines at preventing COVID-19. But it's unclear whether differences between the shots, or exposure to more elusive variants in testing, are the cause.
By Ben Fidler • Updated June 17, 2021 -
Biogen gene therapy deal has yet to bear fruit
Another gene therapy from Nightstar Therapeutics, which Biogen bought for $800 million in 2019, has failed in a key clinical study.
By Jacob Bell • June 15, 2021 -
Former FDA chief Hahn joins venture firm that launched Moderna
Hahn's appointment as chief medical officer of Flagship Pioneering is another example of FDA officials joining industry after their government service. Hahn's predecessor, Scott Gottlieb, sits on Pfizer's board of directors.
By Jonathan Gardner • June 15, 2021 -
Sage antidepressant succeeds in key study, but data raise questions
Zuranolone, a drug that's crucial for Sage's future and the focus of a multi-billion dollar bet by Biogen, could be headed for a regulatory review. But new trial results are more mixed than the overall outcome suggests.
By Ben Fidler , Ned Pagliarulo • Updated June 15, 2021 -
Moderna founder raises another $2B to fuel new startups
Flagship Pioneering reopened one of its funds in April, quickly raising fresh cash for new biotech investments.
By Jacob Bell • June 14, 2021 -
Blood disease treatment from CRISPR, Vertex shows promise in more patients
The latest results from the companies' trials give support to hopes that CRISPR gene editing could functionally cure sickle cell disease and beta thalassemia.
By Ned Pagliarulo • June 11, 2021 -
Vertex stops work on second rare disease drug after study results disappoint
The biotech said its drug for alpha-1 antitrypsin deficiency wasn't potent enough to advance into further testing, but plans to move others into clinical trials next year.
By Jacob Bell • Updated June 11, 2021 -

 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
Beigene expands into cancer cell therapy with Shoreline pact
The fast-growing biotech will work with Shoreline to develop cancer treatments using natural killer cells, an emerging alternative to T-cell based therapies.
By Ned Pagliarulo • June 9, 2021 -
Deep Dive // New Alzheimer's drugs
A first-of-its-kind Alzheimer's drug raises heavy questions around who will and won't get it
Biogen priced its newly approved medicine Aduhelm at an average cost of $56,000 a year, adding affordability to other barriers patients may face.
By Jacob Bell • June 8, 2021 -
The FDA approved Biogen's Alzheimer's drug. The company now has years to confirm it works.
Officially, Biogen has until 2029 to complete a study confirming Aduhelm's benefit. But the agency says it hopes to push the drugmaker to finish faster.
By Ned Pagliarulo • Updated June 9, 2021 -
Deep Dive // New Alzheimer's drugs
In historic move, FDA approves a closely watched and controversial Alzheimer's drug
The decision cleared the way for what many predicted would become a lucrative treatment. But a high price and controversy over whether Biogen's Aduhelm benefits patients weighed heavily on the drug's launch.
By Jacob Bell • Updated June 7, 2021 -
Bluebird cleared by FDA to resume studies of sickle cell gene therapy
An investigation by the company determined the treatment was "very unlikely" to be the cause of a case of leukemia reported in one of the trials.
By Ned Pagliarulo • June 7, 2021 -
Orchard abandons promising gene therapy for rare immune disorder
The U.K. biotech deprioritized the therapy last year, despite positive results in clinical testing that were recently published in a top medical journal.
By Kristin Jensen • June 3, 2021 -
Biotech venture capital still booming as venBio raises another $550M
VenBio has previously invested in a range of treatment areas and technologies, including cell and gene editing companies like Precision Bio and Artiva.
By Jacob Bell • June 3, 2021 -
Bayer buys a biotech and its offshoot in bet on radiopharmaceuticals
The German pharma will add an experimental prostate cancer medicine to its pipeline through the deal, which reflects growing industry interest in the field.
By Ned Pagliarulo • June 3, 2021 -
MorphoSys to buy Constellation in $1.7B deal, aided by unusual funding
Royalty Pharma will effectively fund MorphoSys' acquisition of the cancer biotech through a separate agreement to buy MorphoSys' shares of royalties on several marketed and experimental medicines.
By Ned Pagliarulo • June 2, 2021 -
Alkermes prepares for uncertain launch as FDA finally clears schizophrenia drug
Lybalvi is meant to be as effective as marketed antipsychotics but without the weight gain patients typically experience. Alkermes will introduce the drug into a competitive market, however.
By Jacob Bell • June 1, 2021 -
Amgen pays $400M for an eczema drug, putting faith in an old partner
A deal with Kyowa Kirin gives Amgen rights to an experimental treatment aimed at an autoimmune target that has drawn interest from multiple drugmakers.
By Kristin Jensen • June 1, 2021 -
Sponsored by Parexel Biotech
4 insights on decentralized trials for biotechs
Here are four things we have learned about DCTs that biotech companies can consider when designing a decentralized or hybrid trial.
June 1, 2021 -

 Retrieved from National Cancer Institute on September 27, 2019
Retrieved from National Cancer Institute on September 27, 2019
In first, FDA approves KRAS-blocking cancer drug from Amgen
For decades, scientists have tried unsuccessfully to target the KRAS gene, which is often mutated in lung, colon and pancreatic cancers. Lumakras is the first drug proven effective.
By Ned Pagliarulo • Updated May 29, 2021 -
EQRx readies a lower-cost alternative to pricey cancer immunotherapies
The biotech and its partner CStone disclosed Phase 3 results in lung cancer that support approval plans for a drug similar to treatments like Merck's Keytruda, but would be priced at a fraction of the cost.
By Ben Fidler • May 28, 2021