Clinical Trials: Page 18
-
NEJM paper fills in details on ‘remarkable’ CAR-T result in autoimmune disease
The full results spotlight cell therapy’s potential to treat lupus and other inflammatory conditions, but also the problems drugmakers must still solve.
By Ben Fidler • Feb. 21, 2024 -
Bavarian Nordic terminates cancer vaccine work
The Danish company scrapped a vaccine in Phase 1 testing and will exit oncology altogether, focusing instead on infectious disease research.
By Jonathan Gardner • Feb. 21, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineNeuroscience drug development
Enthusiasm is running higher among drugmakers and investors for neuroscience drug development, buoyed by recent approvals of new Alzheimer’s, ALS and depression medicines.
By BioPharma Dive staff -
AstraZeneca gets new Tagrisso OK as drug succeeds in another trial
The FDA approved the targeted therapy together with chemo for first-line metastatic lung cancer, while fresh trial results supported earlier use.
By Ned Pagliarulo • Feb. 20, 2024 -
Sponsored by Allucent
Translating complex data into actionable insights, to create big value for smaller biotech companies
Why clinical pharmacology is critical to accelerating drug development, and how it can help drive value in a complex and rapidly evolving landscape.
Feb. 20, 2024 -
ALS drug development
ALS drug from Denali, Sanofi falls short in mid-stage study
The disclosure the experimental therapy missed its trial goal comes after Sanofi had earmarked the drug for advancement into Phase 3.
By Delilah Alvarado • Feb. 16, 2024 -
Alnylam changes to heart drug trial spark doubts, sinking shares
Analysts pressed Alnylam executives on the reasons for changes to HELIOS-B, a Phase 3 study in ATTR amyloidosis with cardiomyopathy that is important to the company’s future.
By Ben Fidler • Feb. 15, 2024 -
Kalvista pill succeeds in late-stage study for rare swelling disorder
The biotech's drug quickly alleviated symptoms of the swelling attacks associated with hereditary angioedema, supporting the idea it could be an oral alternative to existing medicines.
By Ben Fidler • Updated Feb. 13, 2024 -
CSL heart drug misses goal in large study
The failure of CSL’s plasma-derived infusion, which contains a protein in “good” cholesterol, follows a similar path as pills from Lilly, Merck, Pfizer and Roche.
By Jonathan Gardner • Feb. 12, 2024 -
Takeda speeds narcolepsy drug to late-stage testing
New results have convinced Takeda to “rapidly” pursue a late-stage program for its drug TAK-861 in one type of narcolepsy, while in another the company opted not to continue.
By Jacob Bell • Feb. 9, 2024 -
J&J says top drug prospect works across autoimmune disorders
The pharma disclosed positive results from two studies testing its antibody drug nipocalimab in generalized myasthenia gravis or Sjögren's disease.
By Jacob Bell • Feb. 5, 2024 -
Non-opioid drug from Vertex cuts pain in major trial tests
While the overall results were positive, the drug fell short in both studies on a secondary goal comparing it to a widely prescribed opioid.
By Ned Pagliarulo • Jan. 30, 2024 -
Deep Dive // State of Play
CAR-T for lupus: the ‘tip of the iceberg’ for cell therapy in autoimmune disease
Since a landmark paper in 2022, drugmakers have begun nearly a dozen trials of cell therapies for lupus, with more set to start. Here’s why their efforts are worth watching.
By Ben Fidler • Jan. 30, 2024 -
Bristol Myers gets positive data in subcutaneous Opdivo trial
An under-the-skin Opdivo shot produced similar results to an intravenous formulation in kidney cancer, giving Bristol Myers a chance at sustaining sales past a key patent expiration in 2028.
By Jonathan Gardner • Jan. 29, 2024 -
Sarepta data show new Duchenne drug’s potency, but highlight side effects
A successor medicine to Sarepta's Exondys 51 appeared better at boosting dystrophin protein production, but was associated with electrolyte imbalances.
By Ned Pagliarulo • Jan. 29, 2024 -
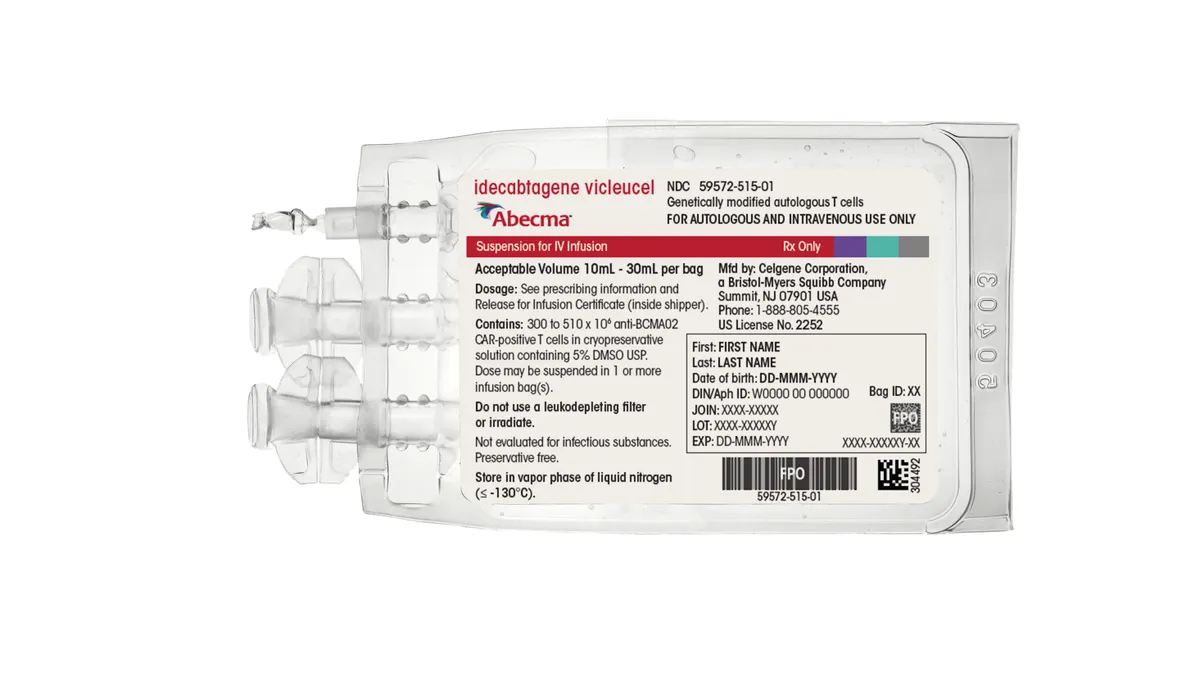
EMA recommends earlier use of Bristol Myers cell therapy for multiple myeloma
The opinion would allow Abecma to be prescribed after two standard therapies and is the latest sign of CAR-T’s growing use in earlier cancer treatment.
By Jonathan Gardner • Jan. 26, 2024 -
Lilly gene therapy finding puts focus on hearing loss treatment pipeline
An 11-year-old boy born deaf can hear after receiving Lilly's genetic medicine. Others, including biotechs in China and Regeneron in the U.S., are taking aim at the same drug target.
By Ned Pagliarulo • Updated Jan. 25, 2024 -
Gilead ADC medicine misses goal in closely watched lung cancer study
Trodelvy didn’t outperform chemotherapy in a large study of people with a common lung tumor, a setback that sent company shares down by double digits.
By Jonathan Gardner • Jan. 22, 2024 -
Ionis says rare disease drug succeeds in late-stage HAE study
The medicine, called donidalorsen, reduced hereditary angioedema-related attacks in the Phase 3 trial. Several competing options to it are already available or in advanced testing, however.
By Ben Fidler • Jan. 22, 2024 -
Novartis details first-line data for radiopharma drug Lutathera
Phase 3 trial results showed Lutathera cut the risk of disease progression or death by 72% as initial treatment for gastroenteropancreatic neuroendocrine tumors, or GEP-NETs.
By Ned Pagliarulo • Jan. 19, 2024 -
Allogene to shift strategy in bid to reposition CAR-T
The company plans to restructure and will start a new late-stage study testing whether its “off-the-shelf” therapy can boost cure rates when used early on.
By Ben Fidler • Jan. 4, 2024 -
Deep Dive
10 clinical trials to watch in the first half of 2024
A non-addictive pain pill faces its definitive test, while study results in ALS and for a Duchenne gene therapy could have far-reaching implications.
By Ben Fidler , Jacob Bell , Ned Pagliarulo , Jonathan Gardner , Delilah Alvarado • Jan. 2, 2024 -
Sarepta tests FDA flexibility with bid to expand Duchenne gene therapy’s approval
The biotech is asking the agency to clear its treatment Elevidys in more patients with the disease, despite a confirmatory trial that missed its main goal.
By Ned Pagliarulo • Dec. 22, 2023 -
Sanofi scraps a top ADC prospect after study setback
The drug, acquired via a deal with ImmunoGen, was the most advanced experimental medicine in the French pharma’s pipeline.
By Ben Fidler • Dec. 21, 2023 -
Argenx autoimmune drug study fails in blow to expansion hopes
The Dutch biotech’s star product has lost some luster after a second failed Phase 3 trial in two months, this time for a skin condition called pemphigus.
By Kristin Jensen • Dec. 20, 2023 -
Deep Dive // Brain drug revival
As ALS research booms, one treatment center finds itself in the spotlight
The Healey center is at the front of ALS research and care, earning acclaim from patients, doctors and scientists. Still, the complexities of the disease and of drug development have brought hard-felt losses.
By Jacob Bell • Dec. 20, 2023