FDA: Page 3
-
Why an FDA decision on Stealth’s Barth drug could ripple through the rare disease field
Stealth secured a new agency review of its experimental therapy elamipretide after a rejection this year. The result could carry broader consequences.
By Alexandra Pecci • Aug. 26, 2025 -
Vaccines
FDA suspends license for Valneva’s chikungunya shot
The agency said it had become aware of more reports of serious adverse events, leading center director Vinay Prasad to conclude the vaccine is no longer safe for its intended use.
By Delilah Alvarado • Aug. 25, 2025 -
FDA’s new accelerated pathway may open pharma up to risks, as well as benefits
Faster review times could leave drugmakers vulnerable to litigation, while new Trump administration priorities add more uncertainty to the approval process.
By Amy Baxter • Aug. 25, 2025 -
Stealth resubmits rare disease drug to FDA
The biotech, which cut jobs following the rejection in May of its Barth syndrome therapy, claimed it has addressed the agency’s concerns and now sees potential for a speedy review.
By Jonathan Gardner • Aug. 18, 2025 -
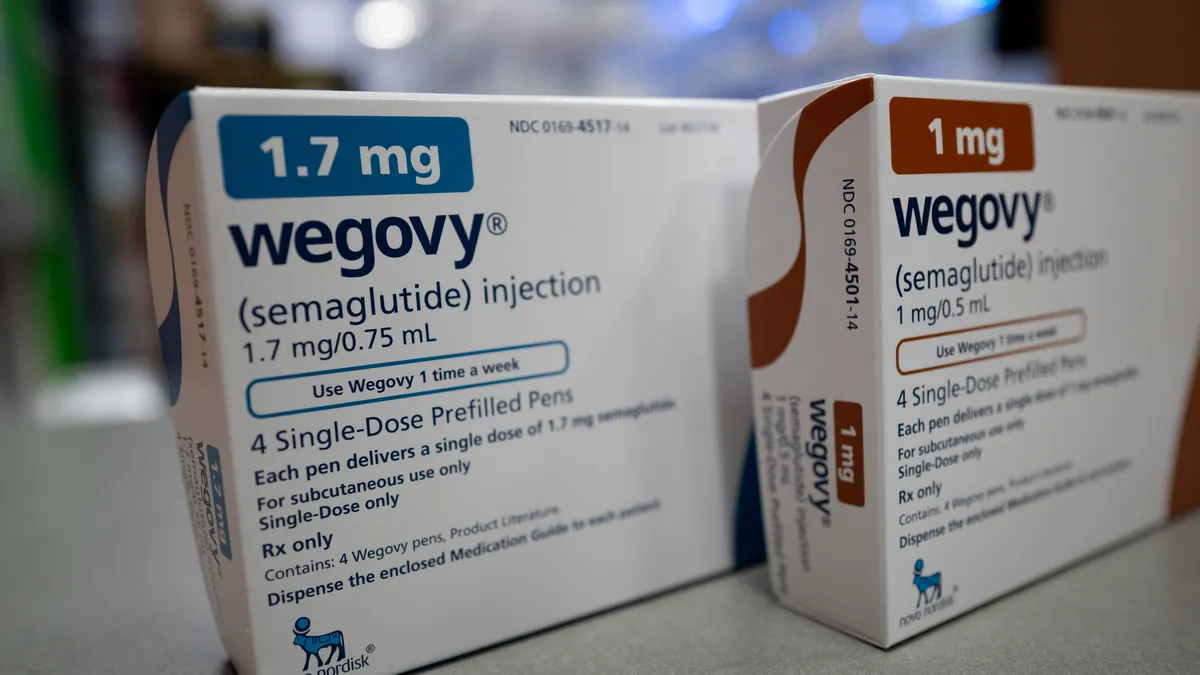
Novo’s Wegovy becomes first GLP-1 drug approved for MASH
The FDA clearance sets Novo’s medicine up for a market battle with Madrigal Pharmaceuticals’ fast-selling Rezdiffra.
By Ben Fidler • Aug. 18, 2025 -
Trump administration
With FDA PreCheck, drugmakers may get a manufacturing boost
The newly announced program targeting domestic manufacturing is the latest Trump administration move to ramp up drugmaking in the U.S.
By Amy Baxter • Aug. 13, 2025 -
Insmed gains US approval of lung disease drug forecast to be blockbuster
Brinsupri, which Insmed acquired from AstraZeneca nearly a decade ago, is the first treatment for bronchiectasis that’s not caused by cystic fibrosis.
By Jonathan Gardner • Aug. 12, 2025 -

 Retrieved from Vinay Prasad on May 08, 2025
Retrieved from Vinay Prasad on May 08, 2025 Trump administration
Trump administrationVinay Prasad, in surprise reversal, to rejoin FDA after abrupt departure
One analyst speculated that, going forward, Prasad may be less “heavy-handed” in reviewing rare disease therapies given the public backlash to the agency’s confrontation with Sarepta.
By Ben Fidler • Updated Aug. 11, 2025 -
Trump administration
FDA to start new ‘precheck’ program to boost US drug production
The initiative is a response to a Trump administration order to speed the construction of the type of new drug factories many pharmaceutical companies have promised to build.
By Ben Fidler • Aug. 7, 2025 -
FDA lifts pause on Valneva’s chikungunya shot, but adds new limits
The FDA had halted use in older adults while it investigated reports of side effects. The vaccine’s label now carries new warnings and restricts vaccination to individuals at high risk of infection.
By Delilah Alvarado • Aug. 7, 2025 -
Trump administration
Vinay Prasad’s ouster leaves biotech guessing at FDA direction
The abrupt exit of the former CBER director raises questions about the FDA’s leadership. For now, newly appointed CDER head George Tidmarsh will take over Prasad’s post.
By Ben Fidler • Updated July 30, 2025 -

 Retrieved from Vinay Prasad on May 08, 2025
Retrieved from Vinay Prasad on May 08, 2025
Vinay Prasad, controversial FDA official, abruptly departs agency
Prasad's exit ends a tumultuous tenure during which he led a reworking of agency guidelines on COVID vaccines and his office got embroiled in controversy over a Duchenne gene therapy.
By Ben Fidler • July 29, 2025 -
FDA allows Sarepta to resume some Elevidys shipments
Wall Street analysts suggested the FDA’s unexpected change in stance might reflect pressure from advocacy groups and a rebuke from higher-ups in the Trump administration.
By Ned Pagliarulo , Ben Fidler • Updated July 29, 2025 -
FDA delays approval decision for Bayer menopause therapy
The agency told Bayer it needs additional time to review the non-hormonal drug, called elinzanetant. Regulators in Canada and the U.K. have already cleared it for use.
By Delilah Alvarado • July 25, 2025 -
FDA panel elevates concerns over antidepressant use during pregnancy
Experts on the panel shared many of the same views around SSRIs, arguing the risks of the drugs during pregnancy are greater than currently accepted.
By Delilah Alvarado • July 22, 2025 -
Sarepta stops Elevidys shipments after standoff with FDA
Company CEO Doug Ingram said the pause was necessary for Sarepta to maintain a "productive and positive working relationship" with the regulator.
By Ned Pagliarulo • July 21, 2025 -
Former biotech executive appointed to lead FDA drug office
George Tidmarsh, a Stanford University physician who founded Horizon Pharma and later ran La Jolla Pharma, was named head of the Center for Drug Evaluation and Research.
By Ned Pagliarulo • July 21, 2025 -
5 questions on Sarepta, the FDA and a Duchenne gene therapy crisis
While Sarepta has now consented to the FDA’s request to stop selling Elevidys, the company’s brief standoff with the agency could still carry major consequences for the Duchenne community.
By Ben Fidler , Ned Pagliarulo • Updated July 22, 2025 -
FDA asks Sarepta to stop shipping Duchenne gene therapy
The company refused the FDA’s request and will continue shipping its therapy, Elevidys, to Duchenne patients who can still walk. A pause on shipments to older, non-ambulatory patients remains in place.
By Ned Pagliarulo • Updated July 19, 2025 -
Panel urges FDA to remove warnings on hormonal menopause therapy
A meeting held by the FDA Thursday spotlighted debate over black box warnings that have limited use of hormone treatment for hot flashes.
By Delilah Alvarado • July 18, 2025 -

 Retrieved from Vinay Prasad on May 08, 2025
Retrieved from Vinay Prasad on May 08, 2025 Vaccines
VaccinesModerna’s latest approval again reveals FDA rift over COVID vaccines
Vinay Prasad overruled other reviewers for a third time in recent months in clearing only narrow use of Moderna's vaccine in young children.
By Delilah Alvarado • July 16, 2025 -
FDA turns back Capricor’s Duchenne cell therapy
The company said it was “surprised” by the decision, which followed the ouster of cell and gene therapy officials from the agency during deramiocel’s review.
By Ben Fidler • July 11, 2025 -
Moderna COVID vaccine gets full approval for children
The approval comes amid regulatory upheaval under HHS head Robert F. Kennedy Jr., who has pushed for changes around mRNA vaccines in particular.
By Delilah Alvarado • Updated July 11, 2025 -
FDA, in policy shift, publishes some drug rejection letters
The agency disclosed a tranche of more than 200 complete response letters from the past five years, but only those involving medicines that it later cleared.
By Ned Pagliarulo • July 10, 2025 -
Novartis gets approval of first malaria medicine for newborns
Coartem Baby, which was cleared by health authorities in Switzerland, will fill an important gap in treatment. Novartis plans to sell it “largely” on a not-for-profit basis.
By Delilah Alvarado • July 8, 2025