Biotech: Page 69
-
ProQR to lay off 30% of staff following eye drug setback
In the near term at least, the biotech company is pinning hopes for its lead eye disease treatment on a new analysis of a recently failed study.
By Kristin Jensen • April 13, 2022 -
Versant-backed startup launches with plans to broaden cell therapy's reach
Cimeio Therapeutics, which emerged Wednesday with $50 million in funding, will use "shielding" technology to try to bring stem cell transplants and adoptive cell therapy to more patients.
By Ned Pagliarulo • April 13, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr.
National Institutes of Allergy and Infectious Diseases. (2016). "Human natural killer cell" [Micrograph]. Retrieved from Flickr. Trendline
TrendlineCell therapy
The continued emergence of CAR-T therapy has fueled research into next-generation approaches and new applications, such as its use in autoimmune diseases.
By BioPharma Dive staff -
UK bid to battle antibiotic resistance yields first subscription-style plan
Pfizer and Shionogi could receive 10 million pounds, or about $13 million, a year for new antibiotics. International participation might be needed to further spur development, however.
By Jonathan Gardner • April 12, 2022 -
AACR 2022: NK cell promise, more TIGIT questions and extending CAR-T's reach
This year's meeting on early cancer research featured promising study results from German biotechs BioNTech and Affimed as well as details from a "practice-changing" lung cancer trial.
By Ben Fidler , Ned Pagliarulo • April 11, 2022 -
Sponsored by Janssen Oncology
[Podcast] Breaking Down Barriers: How Bladder Cancer Innovations Benefit Patients and Treatments for Other Diseases
Recent breakthroughs in bladder cancer treatment are helping patients combat one major challenge to current options — the bladder permeability barrier. These treatments also have implications for other cancers and diseases. This podcast explains how.
By BioPharma Dive's studioID • Updated Feb. 9, 2023 -
Biotech startups face ‘trickle-down effects’ as sector’s IPO drought endures
The slowdown has already forced many biotechs to wait longer to go public. There are early signs private financing deals may be flagging as well.
By Ben Fidler • April 11, 2022 -
Akebia to lay off 42% of workforce, suspend trials after FDA drug rejection
The layoffs come after safety concerns led the FDA to rebuff its anemia pill, similar to the agency's spurning of FibroGen's rival drug last year.
By Ben Fidler • April 7, 2022 -
Alnylam to wait longer for FDA verdict on latest RNA drug
The FDA has yet to sign off on a third-party packaging and labeling facility that Alnylam planned to help launch its drug, called vutrisiran, meaning an approval decision could come as late as mid-July.
By Jacob Bell • April 4, 2022 -
Vertex gets positive trial results for non-opioid painkiller in boost to pipeline
After several attempts at designing a drug for acute pain, Vertex has a candidate it's ready to take into late-stage testing following two successful Phase 2 studies.
By Ned Pagliarulo • March 31, 2022 -
FDA rejects Akebia drug, dealing another blow to anemia pills
The biotech's experimental medicine, meant to be a convenient alternative to widely used injectable drugs like Aranesp, is the second of its kind to be knocked back by the agency due to safety concerns.
By Jonathan Gardner • March 30, 2022 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from Flickr.
Adagio plans comeback for COVID drug, but is it too late?
The biotech said it will ask the FDA for approval of its antibody treatment after positive results from two trials. But relatively few participants were exposed to omicron, potentially raising questions about its utility.
By Jonathan Gardner • March 30, 2022 -
Orchard turns to layoffs in cutting gene therapy research
The biotech company plans to reduce its workforce by 30%, or by about 65 employees, in the latest example of a gene therapy developer restructuring.
By Ned Pagliarulo • March 30, 2022 -
Biogen shelves ALS drug after early-stage trial failure
The study setback raises questions over how well a type of genetic medicine can work in adults with central nervous system disorders like ALS.
By Jonathan Gardner • March 28, 2022 -
As biotech retreats, gene therapy companies retrench and redraw plans
At least nine biotechs working in cell or gene therapy have announced layoffs, cost cuts or restructured their research since December — restructurings that have coincided with a stock market downturn.
By Ned Pagliarulo • March 24, 2022 -
GSK partners with LifeMine as startups advance plans to make drugs from fungi
The deal, announced alongside a $175 million round, makes biotech entrepreneur Greg Verdine's startup the most well-funded among an emerging group of companies searching for drugs in fungal DNA.
By Ben Fidler • March 23, 2022 -
Al Sandrock takes on CEO role at gene therapy developer
The well-known researcher and Biogen alumnus has agreed to take the top spot at Voyager Therapeutics, which, last spring, underwent a "strategic shift" that saw its then-CEO and research head depart.
By Jacob Bell • March 22, 2022 -
Argenx boosted by positive trial results for new shot
An easier-to-take version of Vyvgart could help Argenx expand the drug's use in autoimmune disease, potentially adding to the biotech's appeal as an acquisition target.
By Jonathan Gardner • March 22, 2022 -
Vertex plans path to FDA for top drug prospect
After observing promising results in a small study, Vertex is moving ahead with a pivotal trial for a kidney disease drug that's become a key part of the company's efforts to grow beyond cystic fibrosis.
By Ned Pagliarulo • Updated March 22, 2022 -
FDA approves new epilepsy drug, handing Marinus a long-awaited victory
Nearly 20 years since its founding, Marinus Pharmaceuticals will make the leap to a commercial-stage biotechnology company with the approval of Ztalmy.
By Jacob Bell • March 21, 2022 -
Sponsored by Stevanato Group
How to eliminate design verification failure for your next device
The role of analytical services in preventing the cost and disruption of design verification failure.
March 21, 2022 -
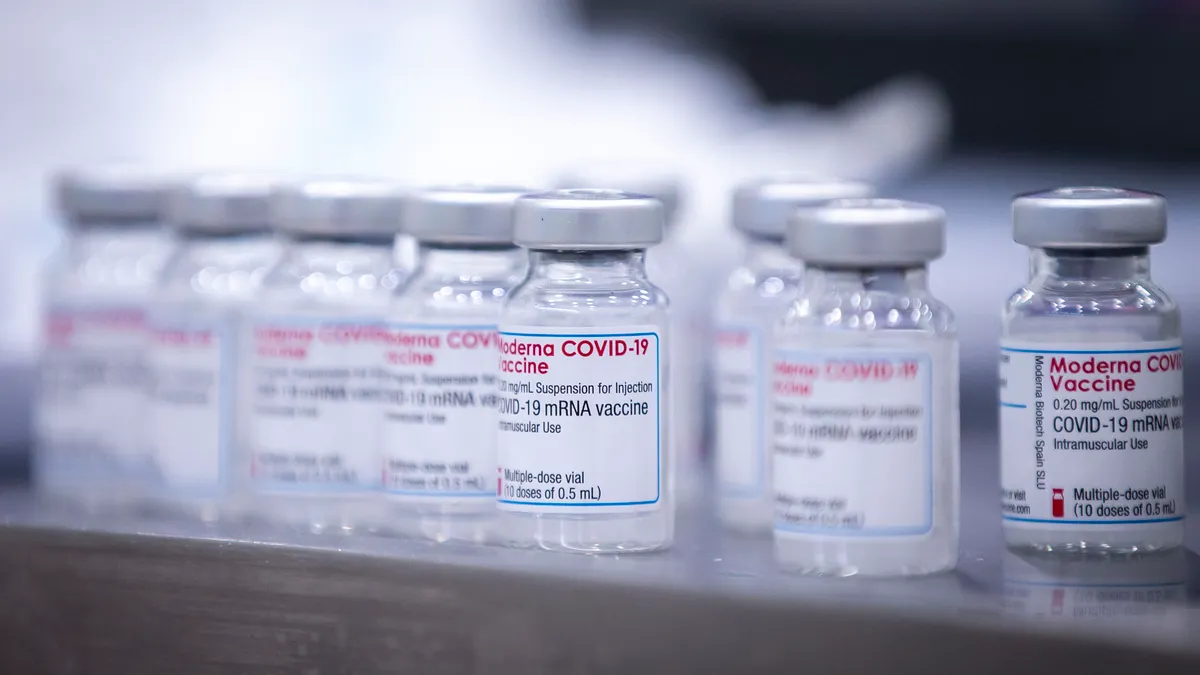
Moderna, citing need for 'flexibility,' seeks broad clearance of fourth COVID shot
Days after Pfizer asked U.S. regulators to authorize another booster for older adults, Moderna has followed with a similar request for all adults, arguing a green light would "provide flexibility" to public health officials and doctors.
By Ben Fidler • March 18, 2022 -
Alnylam sues Pfizer, Moderna over technology used in COVID vaccines
The biotech claims the lipid nanoparticles used by Pfizer and Moderna for delivery of their coronavirus shots infringe on a key patent it holds. In response, Moderna called Alnylam's suit "blatant opportunism."
By Ned Pagliarulo • Updated March 17, 2022 -
Passage Bio to cut jobs in latest biotech restructuring
Stung by a stock market downturn, a number of biotechs are trimming spending and reprioritizing research to save cash. Passage's layoffs will reduce its workforce by 13%.
By Ned Pagliarulo • March 15, 2022 -
BridgeBio looks for comeback with trial results for muscular dystrophy drug
Positive signs for the experimental treatment could help shore up investor confidence in BridgeBio after the December study failure of a drug for a rare heart condition.
By Jonathan Gardner • March 14, 2022 -
Nektar, Bristol Myers drug combination fails in late-stage melanoma trial
The trial results were the most significant setback for the companies' four-year-old partnership and will likely force Nektar to cut costs.
By Ned Pagliarulo • March 14, 2022