Clinical Trials: Page 37
-
Cortexyme plans path forward for Alzheimer's drug that failed study
Detailed trial results presented last week are "interesting," one Alzheimer's expert said, but there are still "many challenges and uncertainties" with Cortexyme's unorthodox approach to treating the disease.
By Ned Pagliarulo • Nov. 12, 2021 -
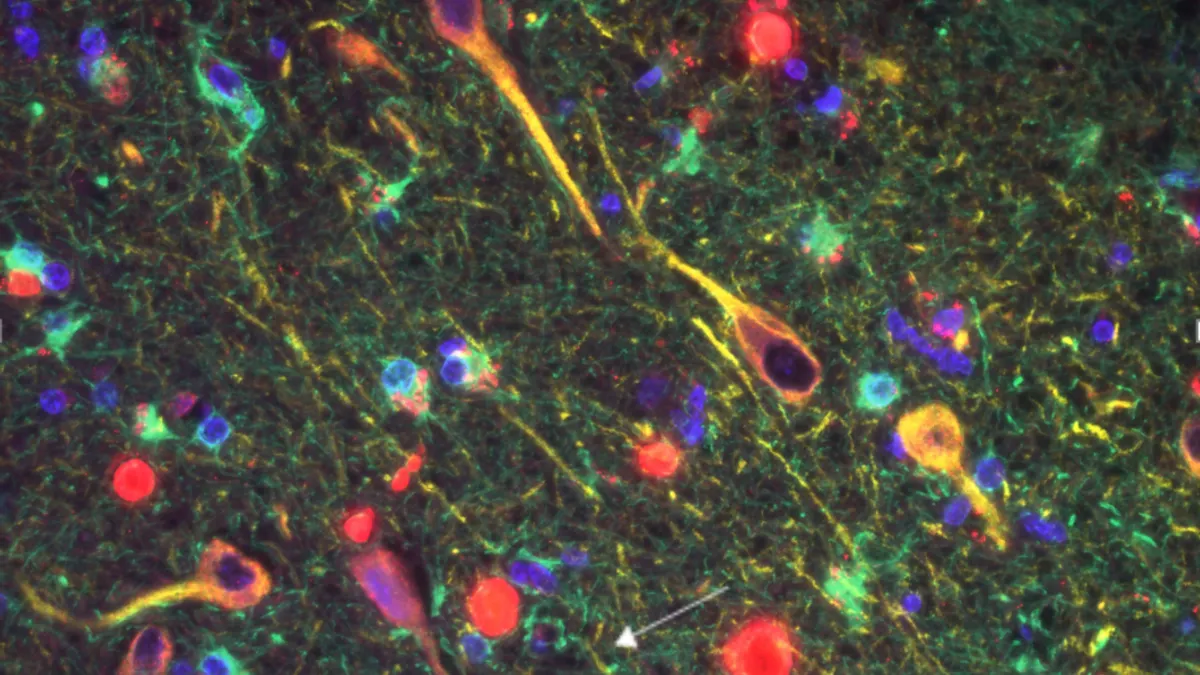
 National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
National Institute on Aging. (2017). "Beta-Amyloid Plaques and Tau in the Brain" [Image]. Retrieved from Flickr.
Detailed Roche study results muddy another Alzheimer's hypothesis
Data presented at an Alzheimer's meeting raised more questions about the seeming benefit of Roche and AC Immune's drug semorinemab, which they claim is the first of its kind to slow deterioration of memory.
By Jonathan Gardner • Nov. 11, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineNeuroscience drug development
Enthusiasm is running higher among drugmakers and investors for neuroscience drug development, buoyed by recent approvals of new Alzheimer’s, ALS and depression medicines.
By BioPharma Dive staff -

 Retrieved from National Cancer Institute on September 27, 2019
Retrieved from National Cancer Institute on September 27, 2019
Mirati gives first look at KRAS drug combination in lung cancer
Study results for Mirati's drug combined with Keytruda have been much anticipated, as the San Diego biotech aims to challenge Amgen and its rival KRAS-blocking drug Lumakras.
By Ned Pagliarulo • Updated Nov. 8, 2021 -
Sponsored by Yourway
Clinical trials face increasing challenges
The COVID-19 pandemic emerged at a time when the clinical trial landscape had reached a peak in complexity. Global trials are now commonplace as drug makers seek to establish efficacy in diverse populations.
By Hussein Pirbhai, Operations Director for UK and Europe, Yourway • Nov. 8, 2021 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49565158908/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49565158908/in/album-72157713108522106/.
Pfizer pill for COVID-19 shows dramatic benefit in major study finding
The drugmaker, which on Oct. 29 won FDA clearance of its vaccine in younger children, plans to quickly ask the agency for emergency authorization of the drug in high-risk patients.
By Jonathan Gardner • Nov. 5, 2021 -
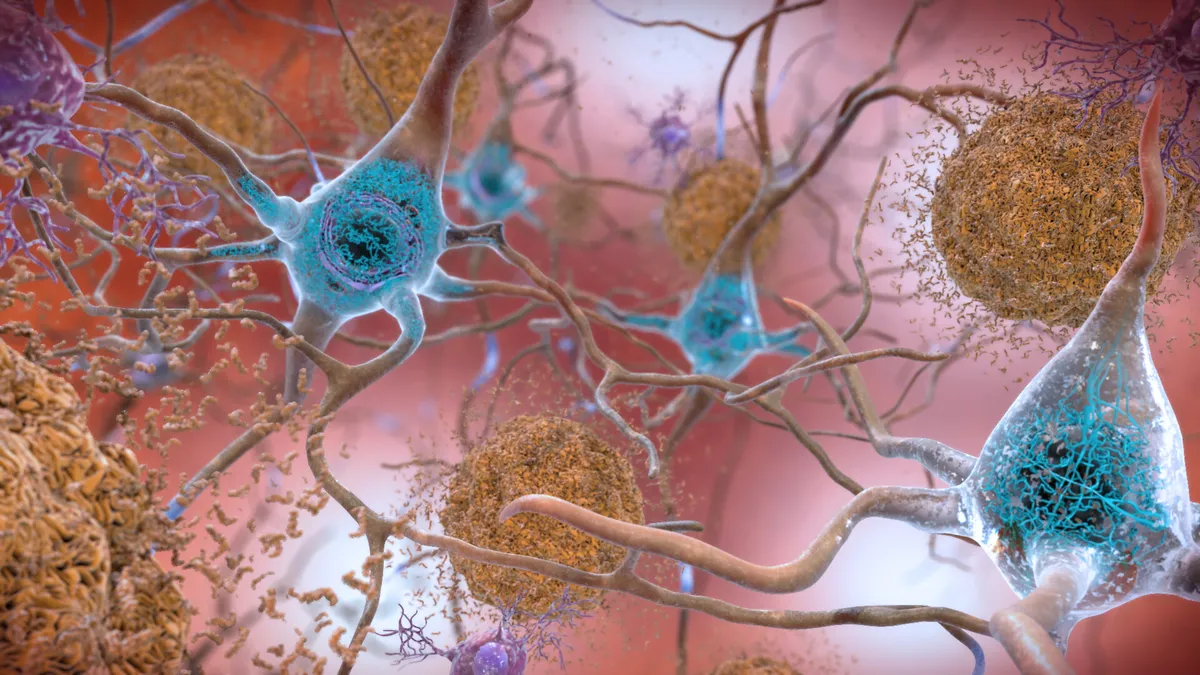
Cortexyme drug fails in Alzheimer's trial, but company sees validation of unorthodox approach
Cortexyme shares lost more than three-fourths of their value after the the biotech's therapy, which is designed to work differently than drugs like Aduhelm, missed both primary goals in a late-stage study.
By Ned Pagliarulo • Oct. 26, 2021 -
Novartis hits another setback in plan to repurpose rare disease drug in lung cancer
Canakinumab, which is approved as Ilaris, didn't slow progression or improve survival when used as a lung cancer treatment. Novartis still hopes it might work alongside surgery.
By Ned Pagliarulo • Updated Nov. 8, 2021 -
Biogen's closely watched ALS drug comes up short in late-stage study
Though the study's primary goal was missed, Biogen claimed there were some signs its drug, tofersen, could slow the disease's progression. The company is now engaging with regulators to discuss tofersen's future.
By Jacob Bell • Oct. 18, 2021 -
Sponsored by Advarra
Why prescreening is the missing piece in a successful participant pipeline
Too often, optimized prescreening is overlooked in favor of the status quo. Here's why that's a mistake—and why prescreening is the great missed opportunity for modern-day trial enrollment.
Oct. 18, 2021 -
 del Aguila III, Ernesto. (2018). "CRISPR Cas9" [Illustration]. Retrieved from Flickr.
del Aguila III, Ernesto. (2018). "CRISPR Cas9" [Illustration]. Retrieved from Flickr.
CRISPR touts new results as fresh questions surround 'off-the-shelf' CAR-T
The biotech's lymphoma treatment initially seems as potent as earlier cell therapies, but its effects waned, adding to doubts about off-the-shelf CAR-T.
By Jonathan Gardner • Updated Oct. 13, 2021 -
Sarepta outlines final push for Duchenne gene therapy
The biotech still believes a speedy approval filing for the closely watched treatment is possible, but expects to have to wait for the results of the recently launched Phase 3 trial, CEO Doug Ingram said on a conference call.
By Ben Fidler • Oct. 11, 2021 -
Sponsored by Clinical Ink
eCOA clinical trials: A simple, cost-effective approach to study build
This new data capture model gives clinical trial sponsors control over study build.
Oct. 11, 2021 -
Relay’s targeted cancer drug could be safer than its competitors. Is it more effective?
Initial study results for Relay's experimental treatment offer some support for the biotech's protein motion technology, but raise some questions, too.
By Ned Pagliarulo • Oct. 8, 2021 -
Allogene cell therapy trials halted by FDA after unexpected safety finding
Researchers found evidence of a "chromosomal abnormality" in one patient treated with Allogene's CAR-T cell therapy, spurring the clinical hold and an investigation.
By Ben Fidler • Updated Oct. 8, 2021 -
Takeda halts studies of touted sleep disorder drug over safety concern
The drugmaker suspended dosing in two mid-stage studies of its experimental narcolepsy treatment after a "safety signal" emerged.
By Ned Pagliarulo • Oct. 6, 2021 -
Collins to step down as NIH head in transition for research agency
After 12 years in the top job, the veteran director says a new scientist should assume leadership of the world's largest biomedical research institution.
By Jonathan Gardner • Oct. 5, 2021 -
J&J heats up RSV race with new vaccine data
The company's candidate is one of two to enter late-stage testing for a respiratory virus that causes hundreds of thousands of hospitalizations a year.
By Jonathan Gardner • Oct. 4, 2021 -
Sarepta embarks on late-stage clinical trial of Duchenne gene therapy
Initiation of the Phase 3 trial is an important milestone for the biotech after earlier setbacks, as well as for patients with the inherited muscle disease.
By Shoshana Dubnow • Oct. 4, 2021 -
Merck says antiviral pill effective against COVID-19, lifting hopes for first oral drug
The drugmaker, along with partner Ridgeback Biotherapeutics, plan to ask the FDA for emergency authorization "as soon as possible" following results so striking that testing was stopped early.
By Ben Fidler • Oct. 1, 2021 -
After long wait, Editas reveals first data for CRISPR gene editing treatment
Early study results offer some hopeful signs but were difficult to interpret. Shares in the biotech slid by nearly 20% in response on Wednesday.
By Ned Pagliarulo • Sept. 29, 2021 -
Sanofi claims positive early data for mRNA COVID shot, but pivots to flu instead
With Pfizer's and Moderna's vaccines broadly used, Sanofi says it will focus on its protein-based coronavirus candidate. The French pharma sees more promise for its mRNA work in influenza.
By Jonathan Gardner • Sept. 28, 2021 -
As FDA scrutinizes immunotherapy approvals, Merck says Keytruda extends survival in liver cancer trial
Phase 3 results showed Merck's drug improved overall survival versus placebo, which should lessen pressure for a market withdrawal in the indication.
By Jonathan Gardner • Sept. 27, 2021 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
Chinese drugmaker Clover says vaccine prevents COVID-19 in large trial
Positive results from Clover's Phase 3 study could mean another coronavirus vaccine option for the many countries still grappling with short supplies.
By Jonathan Gardner • Sept. 22, 2021 -
Pfizer says coronavirus vaccine is safe and spurs immune response in children
The results position Pfizer and BioNTech to seek clearance in children as young as 5 years old. But the companies haven't yet shared data on a rare heart inflammation associated with their shot that's a concern for regulators.
By Jonathan Gardner • Sept. 20, 2021 -
ESMO21: Mirati sets new KRAS bar, AstraZeneca's breast cancer data and Keytruda challengers line up
Mirati's KRAS-blocking drug looks to have an edge over Amgen's, while AstraZeneca and Daiichi reported impressive data for their drug Enhertu.
By Ned Pagliarulo • Updated Sept. 21, 2021