Marketing: Page 16
-
Copycat to top-selling eye drug takes a step toward market
The FDA is reviewing Samsung Bioepis and Biogen's biosimilar of Lucentis. If approved, it would be the first biosimilar for an eye disease drug.
By Kristin Jensen • Nov. 18, 2020 -
Sponsored by IQVIA
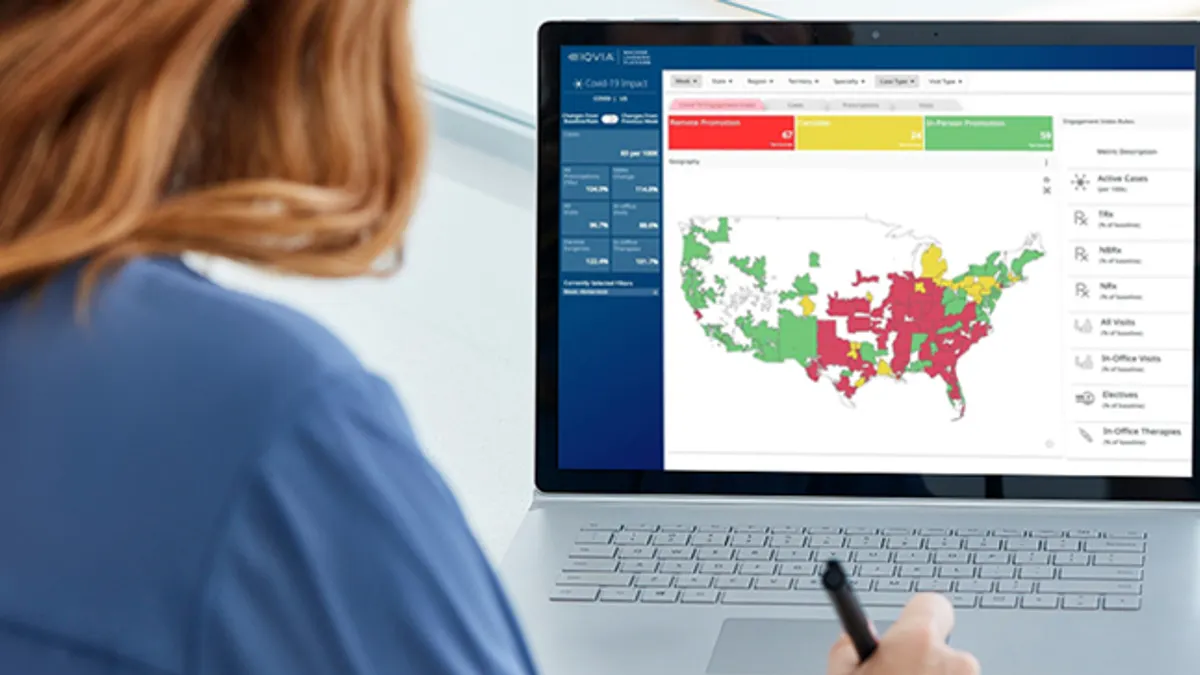
Finding the right mix: Digital transformation offers opportunities for improved HCP engagement
Field teams and HCPs alike are learning how digital, data-driven approaches can best support safe, productive engagement.
Nov. 9, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Brian Tucker / BioPharma Dive/BioPharma Dive
Brian Tucker / BioPharma Dive/BioPharma Dive Trendline
TrendlineCommercialization
New drugs for obesity and Alzheimer’s look set to become blockbusters, reshaping pharma marketing strategies just as many current top-sellers near looming patent cliffs.
By BioPharma Dive staff -
Trump administration to require insurers cover coronavirus vaccines
Late-stage vaccine results are expected soon from Pfizer and Moderna, putting pressure on the Trump administration to put in motion plans for what's likely to be a complex and challenging distribution process.
By Shannon Muchmore • Oct. 29, 2020 -
EQRx builds cancer drug pipeline as plans to challenge big pharma take shape
The startup, which aims to develop lower-cost alternatives to branded medicines, has licensed two drugs from CStone Pharmaceuticals in a deal that could signal a coming price war in cancer immunotherapy.
By Kristin Jensen • Oct. 27, 2020 -
Facing vaccine doubts, US grapples with building confidence in coronavirus shots
The CDC, for example, doesn't support vaccine mandates for healthcare workers or essential employees, an agency official told experts convened by the FDA last week.
By Jonathan Gardner • Oct. 22, 2020 -
Purdue, reaching $8B settlement with US, pleads guilty to charges over opioid sales
Under a deal reached with the Department of Justice, Purdue faces fines and a forfeiture worth more than $5 billion — the largest penalties ever for a drug company.
By Jacob Bell • Oct. 21, 2020 -
Sponsored by Veeva Crossix
Doctors are consumers too! How DTC media impacts HCPs
The findings have implications for how pharma teams should think about their DTC and HCP efforts — they are no longer two separate marketing channels.
Oct. 19, 2020 -

 Retrieved from Nestle on January 16, 2020
Retrieved from Nestle on January 16, 2020
Nestlé makes newly acquired Aimmune a stand-alone unit, appoints new CEO
Andrew Oxtoby, formerly chief commercial officer at the peanut allergy drugmaker, will take over as head of the new Nestlé business.
By Kristin Jensen • Oct. 14, 2020 -
FDA moves to pull preterm birth drug from market
AMAG Pharma, the maker of Makena, said the company disagreed with the FDA's proposal and plans to evaluate its options following a failed study.
By Kristin Jensen • Oct. 7, 2020 -
Sponsored by IQVIA
Achieving success in pharma marketing: The need for a personalized, omnichannel approach
Personalized, insight-driven omnichannel marketing has never been more beneficial or important.
Oct. 7, 2020 -
After setback in the US, Galapagos' main drug finds success elsewhere
A month after the FDA surprisingly rejected the rheumatoid arthritis drug, called filgotinib, it's been cleared by regulators in Europe and Japan.
By Jacob Bell • Sept. 28, 2020 -
Health insurer sues Indivior over 'profit protection scheme'
Centene aims to recoup money it paid for Indivior's Suboxone product, alleging the drugmaker devised a plan to box out generic competitors.
By Samantha Liss • Updated Sept. 23, 2020 -
Cigna rebrands health services division, including Express Scripts, as Evernorth
The change, which has been in the works since before the pandemic, is the next step in integrating the companies' business since their merger in 2018.
By Rebecca Pifer • Sept. 16, 2020 -
Hospitals urge HHS to step in on 340B fight with drugmakers
The dispute stems from drugmakers deciding to stop giving 340B discounts to contract pharmacies, which many hospitals use.
By Shannon Muchmore • Sept. 9, 2020 -
Thermo Fisher deal for Qiagen falls apart after companies fail to secure investor backing
Thermo Fisher had raised its bid for Qiagen to about $12.5 billion, but less than half of the company's shares were tendered by the offer's expiration Monday.
By Nick Paul Taylor , Maria Rachal , Greg Slabodkin • Updated Aug. 13, 2020 -
Bristol Myers pledges to spend $300M on addressing racial, health disparities
The drugmaker aims to double Black and Latinx representation in its executive ranks, among other commitments.
By Kristin Jensen • Aug. 12, 2020 -
First liquid biopsy test that uses advanced gene sequencing approved by FDA
The test, developed by Guardant Health, combines next-generation sequencing with a cancer blood test.
By Susan Kelly • Aug. 10, 2020 -
Amarin decides to go it alone in Europe, as uncertainty clouds heart drug's US future
Chief executive John Thero said the company decided against "multiple proposals" for sales partnerships on Vascepa in Europe. Doing so, however, keeps the pressure on Amarin to deliver.
By Ben Fidler • Aug. 4, 2020 -
Biogen points to COVID-19 for disappointing drug launch
Sales of Vumerity, a key new product in Biogen's plan to bolster its multiple sclerosis drug business, have been slow since it launched in December.
By Jacob Bell • July 22, 2020 -
Ohio sues Express Scripts for overcharging state pension fund
"We want our money back," attorney general Dave Yost said, arguing Express Scripts "egregiously charged for services it didn't deliver." It's the latest action taken by the state against pharmacy benefit managers.
By Samantha Liss • July 15, 2020 -
AbbVie's 'drip-feed' of patents shields top cancer drug from competition, group says
More than seven dozen patents have been granted for Imbruvica in the U.S., helping AbbVie create a legal defense that could protect the drug until 2036.
By Kristin Jensen • July 15, 2020 -


Shutterstock/YAKOBCHUK VIACHESLAV
 Sponsored by Crossix
Sponsored by Crossix4 months with COVID-19: Impact on US health and media behaviors
To help pharmaceutical companies navigate the volatile industry landscape, Crossix has continued to monitor country-wide health and media behaviors.
July 14, 2020 -
Providers, insurers back ad campaign urging patients to stop 'medical distancing'
Since the pandemic's onset in the United States, health officials have been concerned about the consequences of routine and preventive care being delayed or put off entirely. Providers also fear further revenue loss.
By Shannon Muchmore • July 8, 2020 -
Novartis to pay $678M to settle claims over 'sham' doctor events
The Swiss drugmaker admitted to hosting lavish dinners and events that were intended to convince doctors to prescribe Novartis heart and diabetes drugs.
By Ned Pagliarulo • July 2, 2020 -
Gilead pricing suggests it will make a business out of COVID-19 drug
Even at the lower end of its price range, remdesivir could earn Gilead a billion dollars. How well the drug's price is received will be an important test for other companies working on coronavirus treatments.
By Jacob Bell • June 30, 2020